Abstract
Hepatocellular carcinoma (HCC), one of the leading causes of cancer-related mortality worldwide, is challenging to identify in its early stages and prone to metastasis, and the prognosis of patients with this disease is poor. Treatment options for HCC are limited, with even radical treatments being associated with a risk of recurrence or transformation in the short term. Furthermore, the multi-tyrosine kinase inhibitors approved for first-line therapy have marked drawbacks, including drug resistance and side effects. The rise and breakthrough of immune checkpoint inhibitors (ICIs) have provided a novel direction for HCC immunotherapy but these have the drawback of low response rates. Since avoiding apoptosis is a universal feature of cancer, the induction of non-apoptotic regulatory cell death (NARCD) is a novel strategy for HCC immunotherapy. At present, NARCD pathways, including ferroptosis, pyroptosis and necroptosis, are novel potential forms of immunogenic cell death, which have synergistic effects with antitumor immunity, transforming immune 'cold' tumors into immune 'hot' tumors and exerting antitumor effects. Therefore, these pathways may be targeted as a novel treatment strategy for HCC. In the present review, the roles of ferroptosis, pyroptosis and necroptosis in antitumor immunity in HCC are discussed, and the relevant targets and signaling pathways, and the current status of combined therapy with ICIs are summarized. The prospects of targeting ferroptosis, pyroptosis and necroptosis in HCC immunotherapy are also considered.
Key words: ferroptosis, pyroptosis, necroptosis, HCC, immunotherapy
1. Introduction
Liver cancer is the sixth most frequently diagnosed cancer worldwide and the third most common cause of cancer-related death, with ~906,000 new cases and 830,000 deaths worldwide in 2020 (1). In addition, the incidence and mortality rates of liver cancer are generally higher in men than in women (1). Hepatocellular carcinoma (HCC) accounts for the largest proportion of cases among all liver cancer types, is heterogeneous and imposes a large global socio-economic burden (2). HCC can occur either due to the dedifferentiation of hepatocytes or due to the development of intrahepatic stem cells (3-5), is characterized by difficulties in early detection, is prone to metastasis and is associated with poor prognosis (6). The aggressiveness of HCC is closely related to its degree of differentiation, microvascular infiltration, intrahepatic metastases and satellite lesions, and poorly differentiated HCC is associated with earlier recurrence and poorer prognosis compared with well-differentiated HCC (7).
HCC therapy options include surgical excision, liver transplantation, radiofrequency ablation and microwave ablation for early-stage HCC (8-10). However, 40% of patients already have advanced HCC when they are first diagnosed (11), resulting in limited treatment options, and even radical treatment can still result in recurrence or transformation within a short period, commonly within 1-3 years (12). Although treatments with multi-tyrosine kinase inhibitors (TKIs) have been demonstrated to prolong the overall survival (OS) of patients with HCC (13,14), drug resistance and side effects limit the effect of TKIs (15,16).
The rise in immune checkpoint inhibitors (ICIs) and breakthroughs in immunology studies have sparked an expanding interest in cancer immunotherapy, especially in antibodies against programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) (17). Anti-PD-1 drugs, such as nivolumab and pembrolizumab, are efficient and well-tolerated in patients with advanced HCC and are currently recognized as second-line treatment options for patients with HCC (18,19). A clinical study, IMBrave150, revealed that atezolizumab (anti-PD-L1) in combination with bevacizumab (anti-vascular endothelial growth factor) was more beneficial than sorafenib (SOR) in patients with advanced HCC, and prolonged the median OS time of patients with unresectable HCC (uHCC) (20,21). Compared with SOR, the combination immunotherapy of durvalumab (anti-PD-L1) and tremelimumab (anti-cytotoxic T-lymphocyte associated protein 4) administered in the HIMALAYA study, which likely exerted antitumor effects by activating T cells, exhibited an improved therapeutic effect for uHCC (17,22). In addition, immunotherapy drugs have the advantage that they do not need to be metabolized by the liver, which marks a significant advancement in the management of advanced HCC (23,24). Nevertheless, ICI therapy for HCC still has shortcomings such as a low response rate, high tumor tolerance to ICI therapy and multiple side effects (25-28), leading to the use of ICI therapy in combination with various other therapies.
It has been confirmed that, during the development and progression of HCC, the balance between regulatory cell death (RCD) and cell survival serves a crucial function, and resistance to apoptosis and evasion of cell death is one of the hallmarks of HCC (29). Overcoming or delaying TKI resistance increases tumor cell death (30,31), and inducing inflammatory forms of cell death may enhance the tumor response to ICI treatment (32). Therefore, cell death has emerged as a popular research topic in the treatment of HCC. Notably, considering that resistance to apoptotic RCD is a general characteristic of cancer, non-apoptotic RCD (NARCD) serves a more crucial role during the development of HCC and its response to therapy (29). TKIs and ICIs are both tightly associated with the regulation of NARCD pathways (33,34).
At present, ferroptosis, pyroptosis and necroptosis are three highly studied types of NARCD in HCC development and treatment, and these influence the fate of cells in the liver (35-40). There are some differences and similarities among these three types of NARCD, and the key features of ferroptosis, pyroptosis and necroptosis, including the morphological and biochemical features, key regulators, and related drugs are shown in Table I. Furthermore, the roles of these NARCD pathways in the tumor microenvironment (TME) and tumor immune microenvironment (TIME) are gradually being recognized. Treatments targeting these NARCD pathways in combination with TKI treatments (41-43) or ICI therapies (44-46) exhibit synergistically enhanced anticancer activity compared with single treatment. Treatments targeting these NARCD pathways could exert anticancer effects even in cancer types resistant to TKIs and ICIs (34,47,48). Only a few patients exhibit a response to TKI or ICI treatment alone, while triggering ferroptosis, necroptosis or pyroptosis can alter this response status and improve the response rate of therapy (32,49). Furthermore, ferroptosis, pyroptosis and necroptosis, as three potential novel mechanisms of immunogenic cell death (ICD) (50,51), have been suggested to transform immune 'cold' tumors into immune 'hot' tumors, increasing the sensitivity to ICI therapy, activating CD8+ T cell adaptive immunity and maintaining durable immune memory so that the body gains long-term antitumor immunity (52). Thus, ferroptosis, pyroptosis and necroptosis are considered three novel potential therapeutic targets to improve the treatment outcomes of HCC (49).
Table I.
Morphological and biochemical features, key regulators and related drugs of ferroptosis, pyroptosis and necroptosis (46,49,54,83,107).
| RCD type | Morphological features | Biochemical features | Positive regulators | Negative regulators | Related drugs |
|---|---|---|---|---|---|
| Ferroptosis | Cell membrane rupture and exfoliation, smaller mitochondria, decreased mitochondrial cristae, increased mitochondrial membrane densities, mitochondrial membrane disruption and normal-size nuclei without chromatin condensation | Intracellular iron accumulation, SLC7A11/GSH/GPX4 pathway inhibition, cysteine deprivation and lipid peroxidation | TFR1, TFRC, DMT1 and ACSL4 | GPX4, SLC7A11, NRF2 and p53 | Sulfasalazine, glutamate, SOR, cisplatin, statins, trigonelline, artesunate, doxorubicin and iron |
| Pyroptosis | Cell swelling, membrane rupture, chromatin condensation and cytoplasmic content release | Induction of inflammatory cytokines, activation of caspases, GSDM cleavage, formation of inflammasome, IL-18 and IL-1β release, and regulation of caspase-dependent pathways | Caspase-1, caspase-4, caspase-5, caspase-11 and GSDM | ESCRT-III and GPX4 | Cisplatin, metformin, anthocyanin, DHA, paclitaxel and doxorubicin |
| Necroptosis | Cell swelling, plasma membrane rupture, chromatin condensation, organelle expansion and cytoplasmic content release | RIPK1/RIPK3-mediated phosphorylation and ubiquitination of RIPK1/RIPK3/MLKL, assembly of necrosome, and release of inflammatory cytokines | RIPK1, RIPK3 and MLKL | AURKA and ESCRT-III | Iron, 5-FU,resibufogenin, shikonin, artesunate and SOR |
5-FU, 5-fluorouracil; ACSL4, acyl-CoA synthetase long chain family member 4; AURKA, aurora kinase A; DHA, docosahexaenoic acid; DMT1, divalent metal transporter 1; ESCRT-III, endosomal sorting complex required for transport III; GPX4, glutathione peroxidase 4; GSDM, gasdermin; GSH, glutathione; MLKL, mixed lineage kinase domain-like pseudokinase; NRF2, nuclear factor erythroid 2-related factor 2; RCD, regulatory cell death; RIPK, receptor-interacting protein kinase; SLC7A11, solute carrier family 7 member 11; SOR, sorafenib; TFR1, transferrin receptor 1; TFRC, transferrin receptor.
The present review first investigates the role of ferroptosis, pyroptosis and necroptosis in the TME and TIME of HCC and summarizes the related novel targets and signaling pathways. Subsequently, the current status of targeting ferroptosis, pyroptosis and necroptosis in combination with multiple HCC treatment modalities is described. In particular, the potential applications of targeting ferroptosis, pyroptosis and necroptosis in combination with ICIs to enhance immune efficacy are discussed.
2. Mechanism of ferroptosis
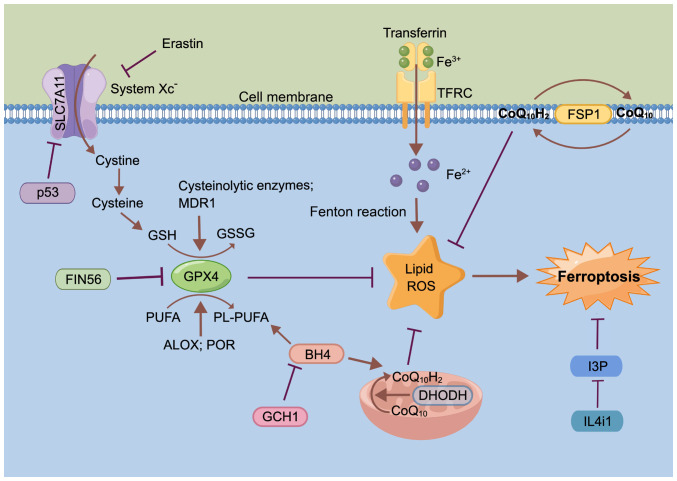
Originally conceptualized in 2012, ferroptosis is considered a lipid peroxidation-driven, iron-dependent and non-apoptotic form of cell death (53). Morphologically, the cellular microstructure after ferroptosis is characterized by organelle expansion, rupture of the plasma membrane and moderate chromatin condensation, and the mitochondrial ultrastructure showing abnormalities including contraction, fracture, enlargement of cristae, increased membrane density and rupture of the outer membrane (53-56). Mechanically, ferroptosis is driven by iron-dependent phospholipid (PL) peroxidation, and regulated by multiple cellular metabolic pathways, including redox homeostasis, iron handling, mitochondrial activity, and metabolism of amino acids, lipids and sugars (Fig. 1) (53,57).
Figure 1.
Overview of the molecular mechanisms of ferroptosis. The figure was drawn using Figdraw (www.figdraw.com). ALOX, arachidonate lipoxygenase; BH4, tetrahydrobiopterin; CoQ10, coenzyme Q10; CoQ10H2, ubiquinol; DHODH, dihydroorotate dehydrogenase; FSP1, ferroptosis suppressor protein 1; GCH1, GTP cyclic hydrolase 1; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, glutathione disulfide; I3P, indole-3-pyruvate; IL4i1, IL-4-induced-1; MDR1, multidrug resistance 1; PL, phospholipid; POR, cytochrome P450 oxidoreductase; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; SLC7A11, solute carrier family 7 member 11; TFRC, transferrin receptor.
Iron and lipid peroxides are important regulators of ferroptosis (53,57). Unstable iron metabolism drives lipid peroxidation to increase susceptibility to ferroptosis, which is in turn closely linked to the inability to store iron during ferritin depletion (58,59). A study suggests that iron storage also requires poly (RC) binding protein 1 to deliver the GSH-iron complex to ferritin, thus repressing ferritinophagy-mediated ferroptosis (60). Furthermore, iron uptake, utilization, storage, and secretion are primarily the responsibility of the liver, which is consequently a central player in iron homeostasis (60,61). Therefore, abnormal iron metabolism is strongly linked to ferroptosis in liver diseases.
Unsaturated fatty acids in cell membranes, such as polyunsaturated fatty acids (PUFAs), are mainly affected by lipid peroxidation driven by free radicals (62). The key characteristic of PUFAs driving ferroptosis is their ability to bind to PLs in membranes upon PUFA activation (62). Arachidonate lipoxygenase and cytochrome P450 oxidoreductase act as regulators of lipid peroxidation, mediating PUFA peroxidation to promote ferroptosis (54,63).
GSH peroxidase 4 (GPX4) acts as a key inhibitor of ferroptosis and is regulated via several mechanisms. For example, ferroptosis inducing 56 can induce degradation of GPX4 to induce ferroptosis (Fig. 1) (64) or covalently binds to the selenocysteine (Sec) site of GPX4 to induce GPX4 inactivation (65). GSH depletion also inactivates GPX4, and thus, drives ferroptosis, while both GSH-related enzymes and the multidrug resistance protein 1, promote GSH depletion (66,67). As research has progressed, a number of novel ferroptosis-inducing mechanisms independent of GPX4 have been identified, such as ferroptosis suppressor protein 1 and dihydroorotate dehydrogenase inhibiting ferroptosis by producing ubiquinol (CoQ10H2) in the cell cytomembrane and inner mitochondrial membranes, respectively (68,69).
Mitochondria serve a diversified role in the process of ferroptosis, iron metabolism and the oxidative phosphorylation pathway, and reactive oxygen species (ROS) are highly involved in ferroptosis (70). It has been demonstrated that the typical metabolic activities of the mitochondria, including tricarboxylic acid cycle and electron transport chain activities, are required for cellular lipid peroxide production in ferroptosis induced by cysteine (Cys) deprivation, but not in that induced by inhibiting GPX4 (57,71).
System Xc-, encoded by the solute carrier family 7 member 11 (SLC7A11) gene, is also a key inhibitor of ferroptosis, and the small molecule erastin and its analogs specifically inhibit cystine uptake through system Xc-, leading to the consumption of GSH and inducing ferroptosis (71). In addition, p53 can also trigger ferroptosis by repressing expression of SLC7A11 and inhibiting cystine uptake through system Xc- (72).
GTP cyclic hydrolase 1 improves ferroptosis sensitivity by inhibiting generation of the antioxidant tetrahydrobiopterin and increasing the abundance of CoQ10H2 and PL-PUFA (73,74). IL-4-induced-1 stimulates ferroptosis by inhibiting production of the metabolite indole-3-pyruvate and limiting the activation of cytoprotective gene expression programs (75).
3. Mechanism of pyroptosis
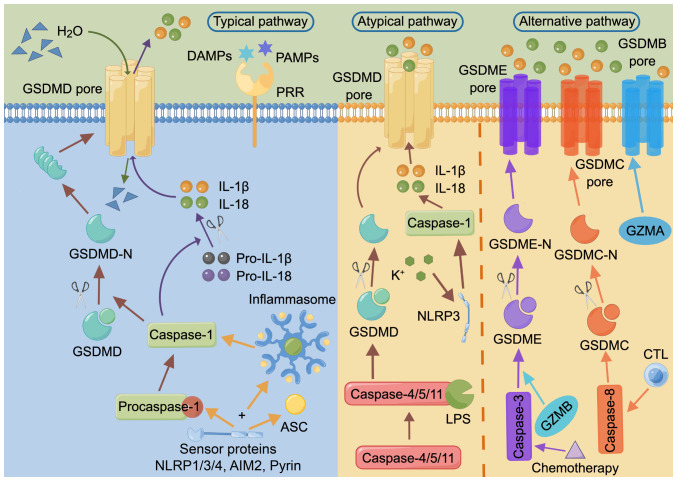
Pyroptosis is a type of programmed cell death mediated by gasdermin (GSDM) (Fig. 2) (76,77). The GSDM family includes GSDMA, GSDMB, GSDMC and GSDMD, as well as two extended family members, GSDME (also referred to as DFNA5) and deafness, autosomal recessive 59 (DFNB59; also referred to as PJVK) (78). Among them, GSDMD, an immediate target of inflammatory caspases and an executor of immune cell pyroptosis, consists of an N-terminal pore-forming domain (PFD) and a C-terminal repressor domain (RD) (76,79,80). N-terminal PFD oligomerization and cell membrane pore formation are regulated by the C-terminal RD (76).
Figure 2.
Summary of three molecular mechanisms of pyroptosis, including the typical, atypical and alternative pathway. The figure was drawn using Figdraw (www.figdraw.com). AIM2, absent in melanoma 2; ASC, apoptosis associated dot like protein; CTL, cytotoxic T lymphocyte; DAMP, damage-associated molecular pattern; GSDM, gasdermin; GZM, granzyme; LPS, lipopolysaccharide; -N, N terminal; NLRP, NOD-like receptor family pyrin domain containing; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor.
The typical pathway of pyroptosis is that, upon host stimulation, GSDMD is cleaved by caspase-1, followed by oligomerization and the formation of functional pores in the cell membrane, leading to the release of inflammatory molecules such as IL-1β and IL-18, disruption of osmotic pressure, water influx, and thus, cell swelling, formation of membrane vesicles with bubble-like protrusions (also known as scorch bodies) and plasma membrane cleavage, and ultimately pyroptosis (80-83).
The atypical pathway of pyroptosis is a process not reliant on inflammasome sensors, and involves the lytic apoptosis of GSDMD cleaved by caspase-4/5/11 interacting with stimulators such as lipopolysaccharide (LPS). The subsequently released K+ activates NOD-like receptor family pyrin domain containing 3 (NLRP3), which in turn activates caspase-1, and thus, indirectly induces IL-1β and IL-18 production (84-86).
In addition, there are alternative pathways of pyroptosis, such as via activated caspase-3/8, that can mediate the cleavage of GSDME or GSDMC, releasing the N-terminal PFD and eventually inducing pyroptosis (87-91). Alternatively, granzyme B from lymphocytes induces pyroptosis by activating caspase-3 and subsequent GSDME cleavage or by directly cleaving GSDME (92,93). Most GSDMs (except DFNB59) have an N-terminal PFD and a C-terminal RD (81), and thus, also have the potential to undergo cellular scorching, with GSDMB, GSDMC and GSDME being the executors of cancer cell pyroptosis (CCP) (78). For example, GSDME can be cleaved by small molecule kinase inhibitors or following the chemotherapy-induced activation of caspase-3, resulting in pyroptosis and the improvement of lung cancer and melanoma treatments (87,94). GSDMC can be cleaved by caspase-8 to trigger CCP, including in lung and liver cancer (88). GSDMB can be cleaved directly by granzyme A released from natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) independently of caspases, contributing to the necrosis of murine cancer cells (95).
In the induction of pyroptosis, inflammasomes are oligomeric complexes composed of sensor proteins, bridging proteins and effector caspases (96,97), and similarly serve as molecular platforms for the activation of inflammatory caspases (97). Pattern recognition receptors (PRRs) can act as sensor proteins, recognizing the relevant molecular patterns that initiate inflammasome activation (98). Among them, NLRP1/3/4, absent in melanoma 2 and pyrin can recruit the adapter apoptosis-associated speck-like protein containing a caspase recruitment domain and/or pro-caspase-1 to assemble into inflammasomes and thereby activate caspase-1, which in turn induces IL-1β and IL-18 processing (99,100).
4. Mechanism of necroptosis
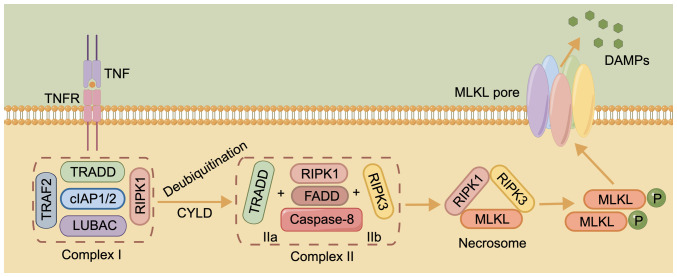
The initiation of necroptosis relies on specific death receptors (DRs), including FAS, tumor necrosis factor receptor 1 (TNFR1) and TNF-related apoptosis-inducing ligand (TRAIL) receptors TRAIL-R1 and TRAIL-R2, or PRRs, such as toll-like receptor (TLR)3, to recognize unfavorable signals from the intra- and extracellular microenvironment (101). TNF is a major stimulus in necroptosis, delivering cell death signals via binding to DRs (102). This process is associated with complex I, consisting of TNFR1-associated death domain protein (TRADD), linear ubiquitin chain assembly complex, TNF receptor-associated factor 2 (TRAF2), cellular inhibitor of apoptosis protein (cIAP)1/2 and receptor-interacting protein kinase (RIPK)1 (103). When cylindromatosis deubiquitinates RIPK1, A20, ubiquitin-specific peptidase (USP)21 or USP20, complex I becomes unstable, and TRADD and RIPK1 are isolated and assembled with Fas-associated death domain (FADD) protein and caspase-8 to form complex IIa, or RIPK3 replaces TRADD to form complex IIb with the other components (104,105). Inactivation of caspase-8 and cIAP induces the conversion of complex IIb into a necrosome, triggering DR-induced necroptosis (106,107).
The necrosome is formed by binding of RIPK3 and RIPK1 via the RIP homotypic-interacting motif (RHIM) domain at first, followed by recruitment of mixed lineage kinase domain-like pseudokinase (MLKL) after the formation of the complex (108). Phosphorylated MLKL, the main executor of necroptosis, later oligomerizes and migrates to the plasma membrane, damaging it and releasing potential damage-associated molecular patterns to trigger necroptosis (Fig. 3) (109,110). Another TLR containing RHIM, TIR domain-containing adapter-inducing interferon-β enables direct RHIM-dependent signaling, initiating necrosis via receptor interacting protein 3 and MLKL (111). A recent study found that extracellular osmotic pressure was also a stimulus for the induction of necroptosis and that the activation of RIPK3 by the Na+/H+ exchanger solute carrier family 9 member A1 increased the cytosolic pH, and this is a pathway that does not depend on the RHIM structural domain to activate the downstream effector MLKL (112).
Figure 3.
Core molecular mechanisms of necroptosis. The figure was drawn using Figdraw (www.figdraw.com). cIAP1/2, cellular inhibitor of apoptosis protein 1/2; CYLD, cylindromatosis; DAMP, damage-associated molecular pattern; FADD, Fas-associated death domain; LUBAC, linear ubiquitin chain assembly complex; MLKL, mixed lineage kinase domain-like pseudokinase; P, phosphate group; RIPK, receptor-interacting protein kinase; TNFR, tumor necrosis factor receptor; TRADD, TNFR1-associated death domain protein; TRAF2, TNF receptor-associated factor 2.
5. Ferroptosis in HCC
As research regarding ferroptosis progresses, more ferroptosis-related drugs and targets are being identified for HCC treatment. As a type of TKI, SOR also induces ferroptosis to augment anti-HCC benefits (113,114). Furthermore, SOR attenuates the binding of beclin-1 to MCL1 by modulating the Src homology region 2 domain-containing phosphatase-1/STAT3 axis, whilst enhancing the binding to SLC7A11 and thereby inhibiting system Xc- activity (115). In addition, glutaminase 2 exerts anti-HCC effects by depleting glutamine, and thus, promoting ferroptosis (116). Using CRISPR screening, phosphatidylserine-transfer RNA kinase depletion has been identified to interfere with Sec and Cys synthesis, leading to GSH depletion and GPX4 inactivation, thus inducing ferroptosis and enhancing the sensitivity to targeted chemotherapy in HCC treatment (117). The long non-coding RNA HEPFA promotes erastin-induced ferroptosis by mediating the destabilization of SLC7A11 through ubiquitination, causing its depletion and the consequent accumulation of ROS and iron (118). In addition, suppressor of cytokine signaling 2 can enhance the degradation of K48-linked polyubiquitinated SLC7A11, and thus, promote ferroptosis to enhance the sensitivity of HCC radiotherapy (119). Ferroptosis inducer erastin and photosensitizer are ultrasonically treated with CD47-transfected donor cells to form engineered exosomes to cause ferroptosis in HCC and avoid phagocytosis by the mononuclear phagocyte system for improved anticancer effects (120). In a previous study, since arsenic trioxide (ATO) could induce ferroptosis, the therapeutic effect of ATO was enhanced by the construction of arsenic-loaded mimetic iron oxide nanoparticles, specifically magnetic nanoparticles containing ATO that were camouflaged with HCC cell membranes (121). In another study, a novel cascade of copper-based nanocatalysts, which can result in ferroptosis alone, also enhanced the HCC treatment effects of cyclooxygenase-2 inhibitor meloxicam and SOR (122). It has also been reported that the pH sensitivity of liposomal vesicles is enhanced following incubation with amphiphilic dendrimers, thereby improving the delivery of the anticancer drug SOR and the ferroptosis inducer hemin in the acidic TME, synergistically treating the induction of ferroptosis and apoptosis (123).
In addition to the previous understanding that SOR induces the death of hepatoma cells, investigations have also revealed that SOR could trigger ferroptosis in hepatic stellate cells (HSCs), as demonstrated in an analysis of liver tissue HSCs from patients with advanced fibrotic HCC treated with SOR monotherapy (124,125). Liver fibrosis is a frequent pathological process that numerous chronic liver diseases undergo before progressing to cirrhosis and HCC, and the conversion of quiescent, vitamin A-storing cells into proliferating, fibrotic myofibroblasts by activated HSCs is a critical step in the development of hepatic fibrosis; therefore, targeted removal of HSCs is of great therapeutic significance (126). ELAV-like RNA binding protein 1, zinc finger protein 36 and N6-methyladenosine can act as regulators of SOR-induced ferroptosis in HSCs (124,125,127). In addition, artesunate reduces hepatic fibrosis by triggering ferroptosis in activated HSCs (128). Furthermore, SOR and artesunate induce ferroptosis through different pathways, and their combined use greatly improves the therapeutic effect in HCC (128). With the rising development of ICIs, SOR has often been used to assess the effectiveness of ICIs in treating HCC, such as in the phase III IMbrave150 and HIMALAYA trials, which demonstrated that the efficacy of ICI combination therapy was comparable to or better than that of SOR in patients with advanced HCC (21). Furthermore, clinical trials of ICIs in combination with SOR (such as NCT03439891 and NCT02988440) are underway and may provide novel perspectives on the administration of ICIs in combination with TKIs.
Given the notable anticancer effect of SOR, there has been an increasing number of studies on the association between ferroptosis and SOR resistance in HCC. For instance, it has been found that Yes1 associated transcriptional regulator (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) and activating transcription factor 4 (ATF4) drive resistance to SOR in HCC by increasing SLC7A11 expression and preventing ferroptosis, and knockdown of YAP/TAZ expression helped to overcome the SOR resistance in HCC (30). Additionally, in SOR-resistant HCC cells, ETS proto-oncogene 1 increases the transcription of microRNA (miR)-23a-3p, which could directly target the 3'-untranslated region of acyl-CoA synthetase long chain family member 4 (ACSL4), the key positive regulator of ferroptosis (47). The co-delivery of ACSL4 small interfering RNA and miR-23a-3p inhibitor abolishes the SOR response (47). Furthermore, metallothionein-1G has been found to facilitate SOR resistance through inhibition of ferroptosis (129). The genetic and pharmacological inhibition of MT-1G enhances the anticancer activity of SOR by inducing ferroptosis of HCC cells (129). A novel photoactive SOR-ruthenium (II) complex, is irradiated to reduce SOR resistance in HCC by inducing ferroptosis and disrupting purine metabolism (31). It has also been found that treatment of HCC with SOR induces macropinocytosis, which replaces ferroptosis-depleted Cys, and thus, promotes resistance to SOR, while amiloride can target micropinocytosis (130).
The proliferation of cancer cells depends on lactic acid, which is produced following glucose consumption via aerobic glycolysis to provide energy and is closely associated with ferroptosis (131). For instance, the glycolytic enzyme α-enolase 1 protects cancer cells from ferroptosis by reducing mitochondrial iron accumulation through inhibition of the iron regulatory protein 1/mitoferrin-1 pathway (132). Monocarboxylate transporter 1 (MCT1)-mediated lactic acid uptake promotes ATP production and AMP-activated protein kinase inactivation in HCC, which in turn upregulates downstream stearoyl-coenzyme A desaturase-1 to enhance the production of anti-ferroptosis monounsaturated fatty acids (133). Hypoxia-inducible factor-1α also drives resistance to ferroptosis in solid tumors by promoting lactate production (134).
Nuclear factor erythroid 2-related factor 2 (NRF2) has long been known to influence tumor progression as a pivotal regulator of the antioxidant response (135,136). Nuclear accumulation of NRF2 has been found to activate ferroptosis-associated proteins, while Kelch-like ECH-associated protein 1 (Keap1), an adaptor of the Cul3-ubiquitin E3 ligase complex, is responsible for the degradation of NRF2; in turn, phosphorylated p62 binds Keap1 with high affinity, thus targeting the p62/Keap1/NRF2 pathway increases the anticancer activity of elastin and SOR, a process that is facilitated by disulfiram/Cu (137-139). The GSH S-transferase Z1/NRF2/GPX4 axis and the leukemia inhibitory factor receptor/NF-κB/lipocalin-2 axis can also be targeted to promote ferroptosis, thereby enhancing the sensitivity to SOR in HCC treatment (140,141).
6. Pyroptosis in HCC
Miltirone, a phenanthrene derivative from the roots of Salvia miltiorrhiza Bunge, and ATO nanoparticles (142) have both been demonstrated to induce GSDME-associated HCC pyroptosis by activating caspase-3, while cannabidiol from the plant Cannabis sativa inhibits aerobic glycolysis via the ATF4/insulin-like growth factor binding protein 1/Akt signaling pathway (143). GSDMD is a common executor of pyroptosis, and mallotucin D activates caspase-9 and caspase-3 in HepG2 cells, inducing GSDMD cleavage and ultimately causing pyroptosis (144). NLRP3, a member of the PRR family, induces inflammasome activation and thus pyroptosis, while metformin indirectly activates NLRP3 and thus pyroptosis in HCC by upregulating FOXO3 (145). The small nucleolar RNA hostgene 7/miR-34a/sirtuin 1 signaling pathway also induces NLRP3-dependent pyroptosis in HCC (146). In addition, 17β-estradiol, alpine ginseng flavonoid and hepatitis C virus infection in Huh-7.5 cells can induce caspase-1-dependent pyroptosis via activation of the NLRP3 inflammasome (147-149). Cisplatin-induced activation of the NLRP3 inflammasome can be inhibited by incomplete radiofrequency ablation-induced upregulation of heat shock protein (HSP) 70, which leads to cisplatin resistance in HCC (150).
7. Necroptosis in HCC
Necroptosis serves an important role in HCC development. According to a previous study, the hepatic microenvironment epigenetically shapes lineage commitment of liver tumorigenesis, and abnormal activation of hepatocyte oncogenes can lead to cholangiocarcinoma if adjacent hepatocytes undergo necroptosis, but hepatocytes regulated by the same oncogenic factors can develop into HCC cells if they are surrounded by apoptotic hepatocytes (151). Deletion of RIPK1, a central element of necroptosis, in hepatocytes induces downregulation of TRAF2, leading to impaired caspase-8 activation and NF-κB activation; therefore, the RIPK1/TRAF2/caspase-8 pathway has a notable influence on the development of HCC (152). As confirmed by a previous study, the NF-κB signaling pathway is an important player in necroptosis (153). Apigetrin and deferasirox exert anti-HCC effects by inhibiting the NF-κB signaling pathway to induce necroptosis (43,154). HSP90α has been found to bind with the necrosome complex and promotes chaperone-mediated autophagy degradation, which leads to necroptosis blocking and results in SOR resistance (48). The HSP90 inhibitor 17-allylamino demethoxygeldanamycin could inhibit HSP90α activity and reverse SOR resistance in HCC by activating necroptosis (48). FADD, RIPK1, RIPK3 and MLKL are key signaling molecules in necroptosis, and miR-675 could target FADD to induce necroptosis and inhibit HCC progression via the RIPK3/MLKL axis (155), and rapamycin could induce HCC cell necroptosis via the RIPK1/RIPK3/MLKL signaling pathway (156). A recent study has found that, as the liver progressively ages, necroptosis increases, which in turn continuously exacerbates chronic liver inflammation, thus exacerbating the HCC transition (157). This necroptosis-mediated liver inflammation process may be prevented by β-carotene and heterogeneous nuclear ribonucleoprotein A1 (158,159). In addition, excess sorbitol dehydrogenase (SORD) in HCC cells could inhibit tumor growth and stemness by enhancing necroptosis signaling, and treatment with human recombinant SORD controlled HCC cell growth and regulated macrophage polarization in the tumor microenvironment (160). Nuclear protein 1 (NUPR1) and Linc00176 are also associated with HCC cell necroptosis, and inhibition of NUPR1 with small compound ZZW-115 and deletion of Linc00176 could induce necroptosis and exert anti-HCC effects (161,162). In addition, connexin 32 has been found to bind to Src and then mediate the inactivation of caspase 8 to trigger necroptosis in HCC cells, which could be used as an anticancer target to enhance the function of necroptosis inducers (163).
8. Ferroptosis, pyroptosis, necroptosis and tumor immunity
During the treatment of tumors, ferroptosis, pyroptosis and necroptosis are closely associated with the immune response (50,95,164). It has been found that immunotherapy-activated CD8+ T cells could induce ferroptosis of tumor cells by downregulating the expression of SLC3A2 and SLC7A11, and ferroptosis inducers in combination with checkpoint blockade synergistically enhanced antitumor efficacy (164). Similarly, CD8+ T cells have also been found to trigger tumor clearance through activation of the GSDM granzyme axis to induce pyroptosis (95,165), as have NK cells (93,95). Furthermore, necroptotic tumor cells can release damage-associated molecular patterns such as heat shock proteins, being more immunogenic than naïve tumor cells, to activate the immune system with the participation of CD8+ T cells, NK cells and dendritic cells (DCs) (166-168), which may be related to the activation of NF-κB (50). Necrotic tumor cells, as well as fibroblast vaccination, contribute to the induction of antitumor immunogenicity by necrotic apoptotic cells (50,167,169), which enhances the antitumor efficacy of ICI treatments (167).
9. Ferroptosis and tumor immunity in HCC
The relationship between ferroptosis and the tumor immune response is complicated. It has been demonstrated that GPX4-associated ferroptotic hepatocyte death could cause a HCC-suppressive immune response, characterized by a C-X-C motif chemokine ligand 10-dependent infiltration of cytotoxic CD8+ T cells that is counterbalanced by PD-L1 upregulation on tumor cells, as well as by a marked high mobility group box 1-mediated myeloid derived suppressor cell (MDSC) infiltration (170). A triple combination of the ferroptosis-inducing natural compound withaferin A, the C-X-C motif chemokine receptor 2 inhibitor SB225002 and α-PD-1 contributed to improved treatment of HCC compared with single treatment or dual combinations (170). In addition, inhibition of phosphoglycerate mutase 1 promotes ferroptosis and downregulates PD-L1 expression in HCC cells, further enhancing the infiltration of CD8+ T cells, and thereby exerting antitumor effects (36). Furthermore, TLR2 agonist Pam3CSK4 could promote polarization of MDSCs into DCs and macrophages and recovery of T cell function by downregulating the expression of RUNX family transcription factor 1 (RUNX1), and TLR2 and RUNX1 may exert a regulatory effect on MDSCs by increasing ROS, which is related to the ferroptosis pathway (171).
Tumor-associated macrophages (TAMs), a key tumor-infiltrating immune cell type in the TME, encourage tumor invasion and metastasis by switching from the pro-inflammatory and antitumor type M1 TAMs to the anti-inflammatory and pro-tumor type M2 TAMs (172). Previous studies have demonstrated that TAMs generally exhibit a pro-tumor phenotype and inhibit CTLs by expressing PD-L1, thereby inducing immune escape and tolerance (173-175). Inhibition of apolipoprotein C1 can reverse the M2 phenotype to the M1 phenotype in TAMs via the ferroptosis pathway, enhancing the effect of anti-PD-1 immunotherapy and exerting anti-HCC effects (176). Injection of dextran chitosan hydrogel can induce the repolarization of macrophages to the M1-like phenotype, and promote the maturation and activation of DCs, and combined treatment with PD-1 immunotherapy can effectively treat the peritoneal dissemination of advanced HCC and malignant ascites (37). A recent study also revealed that inhibition of the heavy chain subunit from system Xc- encoded by the SLC7A11 gene (xCT) could induce ferroptosis of TAMs via the GPX4/ribonucleotide reductase regulatory subunit M2 signaling pathway and inhibit M2-type polarization via the suppressor of cytokine signaling 3/STAT6/peroxisome proliferator-activated receptor (PPAR)-γ pathway (35). Furthermore, this xCT inhibition-mediated macrophage ferroptosis increased PD-L1 expression in macrophages and improved the antitumor efficacy of anti-PD-L1 therapy (35).
10. Pyroptosis and tumor immunity in HCC
TKIs can remodel the TME of HCC and alter the immunosuppressive microenvironment, which is closely connected to pyroptosis, NK cells, T cells and regulatory T cells (177). Among them, SOR is a multi-target kinase inhibitor that directly modulates immunity, causing HCC cell death by inducing macrophage pyroptosis and activating cytotoxic NK cells (41). A study has found that the pyroptosis-score (PYS) could be used to assess the prognosis of patients with HCC due to hepatitis B virus (HBV-HCC), as a higher PYS in patients with HBV-HCC was associated with a worse prognosis, and these patients were more likely to receive anti-PD-L1 therapy (178). In addition, a risk score model related to pyroptosis can be constructed to predict the prognosis and immunological characteristics of HCC (38).
11. Necroptosis and tumor immunity in HCC
The interaction of necroptosis and the immune response is considered to be important in HCC development and treatment because necroptosis is a primary cause of liver inflammation and upregulates not only proinflammatory M1 TAMs, but also proinflammatory cytokines and chemokines, ultimately leading to chronic liver disease (179). However, a study has reported that RIPK3 is not expressed in hepatocytes, and MLKL does not depend on RIPK3 alone to regulate endoplasmic reticulum (ER)-mitochondrial Mg2+ dynamics (108). Defective MLKL inhibits mitochondrial Mg2+ absorption and ER Mg2+ release, and the resulting metabolic stress, in turn, induces parthanatos [a form of ICD associated with antitumor immunity (180)], activates anticancer immune responses and increases the therapeutic impact of ICIs, inhibiting HCC progression and immune escape (181). Similarly, RIPK3 defects in TAMs activate PPAR and promote fatty acid metabolism, which in turn induces the accumulation and polarization of M2 TAMs and ultimately promotes HCC progression (182). In addition, higher expression levels of RIPK1, RIPK3 and MLKL, are associated with good prognosis in HCC and are especially positively associated with CD3+ and CD8+ T cells in HCC (183). However, monoclonal antibodies targeting CD147 structural domain 1 can induce atypical necroptosis independent of RIPK (184). Furthermore, both senescence and SORD induce necroptosis in HCC cells and promote M1 TAM polarization (157,160).
12. Conclusion
In conclusion, induction of ferroptosis, pyroptosis and necroptosis is a novel avenue for killing HCC cells, providing novel targets and signaling pathways for drug discovery, increasing treatment effectiveness of immunotherapy, and possibly addressing drug resistance. The combination of ferroptosis, pyroptosis and necroptosis targets with ICIs may improve the therapeutic effect and prognosis in patients with advanced HCC. However, some problems still remain. First, although several compounds or drugs that induce ferroptosis, pyroptosis and necroptosis have been identified, clinical studies to assess their feasibility and safety are lacking. Therefore, more research should be performed in the future to improve the safety and personalized treatment options of this treatment avenue. Second, clinical studies of novel drugs or therapies for HCC are usually long and costly, which makes it difficult to develop novel medicines and treatments based on ferroptosis, pyroptosis and necroptosis. Third, the significance and mechanisms of ferroptosis, pyroptosis and necroptosis in HCC still need to be elaborated in further detail.
Acknowledgments
Not applicable.
Abbreviations
- HCC
hepatocellular carcinoma
- TKI
tyrosine kinase inhibitor
- ICI
immune checkpoint inhibitor
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- TME
tumor microenvironment
- ICD
immunogenic cell death
- TIME
tumor immune microenvironment
- Cys
cysteine
- ROS
reactive oxygen species
- GSH
glutathione
- PL
phospholipid
- GPX4
glutathione peroxidase 4
- Sec
selenocysteine
- SLC7A11
solute carrier family 7 member 11
- PFD
pore-forming domain
- RD
repressor domain
- CCP
cancer cell pyroptosis
- CTL
cytotoxic T lymphocyte
- PRR
pattern recognition receptor
- TLR
toll-like receptor
- LPS
lipopolysaccharide
- TRADD
tumor necrosis factor receptor 1-associated death domain protein
- RIPK
receptor-interacting protein kinase
- FADD
Fas-associated death domain
- MLKL
mixed lineage kinase domain-like pseudokinase
- RHIM
RIP homotypic-interacting motif
- SOR
sorafenib
- HSC
hepatic stellate cell
- miR
microRNA
- TAM
tumor-associated macrophage
- MDSC
myeloid-derived suppressor cell
- PYS
pyroptosis-score
Funding Statement
The present study was supported by the Fundamental Research Funds for the Central Universities, Beijing University of Chinese Medicine (grant no. 2021-JYB-XJSJJ-055), Beijing Traditional Chinese Medicine 'Torch Inheritance 3+3 Project'-the Wang Pei Famous Doctor Inheritance Workstation-Dongzhimen Hospital Branch (grant no. 405120602) and 'Talent development program of Dongzhimen Hospital, Beijing University of Chinese Medicine'.
Availability of data and materials
Not applicable.
Authors' contributions
RJL and XDY wrote the manuscript. RJL drew the figures. SSY and ZWG participated in the conception of the manuscript. XBZ and YSZ revised the manuscript. Data authentication is not applicable. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F, Vaccarella S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023;11:e197–e206. doi: 10.1016/S2214-109X(22)00501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47:1789–1797. doi: 10.1016/j.ejca.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Park YN. Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med. 2011;135:704–715. doi: 10.5858/2010-0524-RA.1. [DOI] [PubMed] [Google Scholar]
- 5.Trevisani F, Cantarini MC, Wands JR, Bernardi M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis. 2008;29:1299–1305. doi: 10.1093/carcin/bgn113. [DOI] [PubMed] [Google Scholar]
- 6.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: Key pathologic and imaging aspects. Radiology. 2014;272:635–654. doi: 10.1148/radiol.14132361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komuta M. Histological heterogeneity of primary liver cancers: Clinical relevance, diagnostic pitfalls and the pathologist's role. Cancers (Basel) 2021;13:2871. doi: 10.3390/cancers13122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berardi G, Igarashi K, Li CJ, Ozaki T, Mishima K, Nakajima K, Honda M, Wakabayashi G. Parenchymal sparing anatomical liver resections with full laparoscopic approach: Description of technique and short-term results. Ann Surg. 2021;273:785–791. doi: 10.1097/SLA.0000000000003575. [DOI] [PubMed] [Google Scholar]
- 9.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, OLT for HCC Consensus Group Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan T, Xie QK, Lv N, Li XS, Mu LW, Wu PH, Zhao M. Percutaneous CT-guided radiofrequency ablation for lymph node oligometastases from hepatocellular carcinoma: A propensity score-matching analysis. Radiology. 2017;282:259–270. doi: 10.1148/radiol.2016151807. [DOI] [PubMed] [Google Scholar]
- 11.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 12.Xing R, Gao J, Cui Q, Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol. 2021;12:783236. doi: 10.3389/fimmu.2021.783236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 15.Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, Darnell A, Ríos J, Ayuso C, Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Cao Q, Wen W, Wang H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett. 2019;460:1–9. doi: 10.1016/j.canlet.2019.114428. [DOI] [PubMed] [Google Scholar]
- 17.Greten TF, Lai CW, Li G, Staveley-O'Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology. 2019;156:510–524. doi: 10.1053/j.gastro.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 20.Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannini EG, Aglitti A, Borzio M, Gambato M, Guarino M, Iavarone M, Lai Q, Levi Sandri GB, Melandro F, Morisco F, et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI.CA cohort derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers (Basel) 2019;11:1689. doi: 10.3390/cancers11111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greten TF, Abou-Alfa GK, Cheng AL, Duffy AG, El-Khoueiry AB, Finn RS, Galle PR, Goyal L, He AR, Kaseb AO, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J Immunother Cancer. 2021;9:e002794. doi: 10.1136/jitc-2021-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Wang Y, Gao P, Ding J. Immune checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer Lett. 2023;555:216038. doi: 10.1016/j.canlet.2022.216038. [DOI] [PubMed] [Google Scholar]
- 27.Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, Li Z, Traugh N, Bu X, Li B, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyiramana MM, Cho SB, Kim EJ, Kim MJ, Ryu JH, Nam HJ, Kim NG, Park SH, Choi YJ, Kang SS, et al. Sea hare hydrolysate-induced reduction of human non-small cell lung cancer cell growth through regulation of macrophage polarization and non-apoptotic regulated cell death pathways. Cancers (Basel) 2020;12:726. doi: 10.3390/cancers12030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S, Piscuoglio S, Dill MT, Camargo FD, Christofori G, Tang F. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13:e14351. doi: 10.15252/emmm.202114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai Y, Lu N, Luo S, Wang H, Zhang P. A photoactivated sorafenib-ruthenium(II) prodrug for resistant hepatocellular carcinoma therapy through ferroptosis and purine metabolism disruption. J Med Chem. 2022;65:13041–13051. doi: 10.1021/acs.jmedchem.2c00880. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum SR, Wilski NA, Aplin AE. Fueling the fire: Inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 2021;11:266–281. doi: 10.1158/2159-8290.CD-20-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 2023;22:723–742. doi: 10.1038/s41573-023-00749-8. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196. doi: 10.1038/s41392-022-01046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang B, Zhu J, Wang Y, Chen W, Fang S, Mao W, Xu Z, Yang Y, Weng Q, Zhao Z, et al. Targeted xCT-mediated ferroptosis and protumoral polarization of macrophages is effective against HCC and enhances the efficacy of the anti-PD-1/L1 response. Adv Sci (Weinh) 2023;10:e2203973. doi: 10.1002/advs.202203973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Y, Wang Y, Lu Z, Wan J, Jiang L, Song D, Wei C, Gao C, Shi G, Zhou J, et al. PGAM1 inhibition promotes HCC ferroptosis and synergizes with anti-PD-1 immunotherapy. Adv Sci (Weinh) 2023;10:e2301928. doi: 10.1002/advs.202301928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng J, Yang X, Huang J, Tuo Z, Hu Y, Liao Z, Tian Y, Deng S, Deng Y, Zhou Z, et al. Ferroptosis-enhanced immunotherapy with an injectable dextran-chitosan hydrogel for the treatment of malignant ascites in hepatocellular carcinoma. Adv Sci (Weinh) 2023;10:e2300517. doi: 10.1002/advs.202300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Zhang B, Shang Y, Chen F, Fan Y, Tan K. A novel risk score model based on pyroptosis-related genes for predicting survival and immunogenic landscape in hepatocellular carcinoma. Aging (Albany NY) 2023;15:1412–1444. doi: 10.18632/aging.204544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng YL, Wang LX, Li MY, Liu LP, Li RS. Construction and validation of a prognostic signature based on necroptosis-related genes in hepatocellular carcinoma. PLoS One. 2023;18:e279744. doi: 10.1371/journal.pone.0279744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Wang Y, Pan J, Gan L, Xue J. Ferroptosis, necroptosis, and pyroptosis in cancer: Crucial cell death types in radiotherapy and post-radiotherapy immune activation. Radiother Oncol. 2023;184:109689. doi: 10.1016/j.radonc.2023.109689. [DOI] [PubMed] [Google Scholar]
- 41.Hage C, Hoves S, Strauss L, Bissinger S, Prinz Y, Pöschinger T, Kiessling F, Ries CH. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell-mediated cytotoxicity against hepatocellular carcinoma. Hepatology. 2019;70:1280–1297. doi: 10.1002/hep.30666. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L, Cheng Z, Zhang J, Li J, Xu X, et al. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Canc Res. 2023;42:6. doi: 10.1186/s13046-022-02567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhosale PB, Abusaliya A, Kim HH, Ha SE, Park MY, Jeong SH, Vetrivel P, Heo JD, Kim JA, Won CK, et al. Apigetrin promotes TNFα-induced apoptosis, necroptosis, G2/M phase cell cycle arrest, and ROS generation through inhibition of NF-κB pathway in Hep3B liver cancer cells. Cells. 2022;11:2734. doi: 10.3390/cells11172734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, Huang H, Shao F, Liu Z. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 45.Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J, Yang K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35:109235. doi: 10.1016/j.celrep.2021.109235. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34:757–774.e7. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, Chan YT, Tan HY, Zhang C, Guo W, Xu Y, Sharma R, Chen ZS, Zheng YC, Wang N, Feng Y. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:3. doi: 10.1186/s13046-021-02208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Y, Yang Y, Pan D, Ding Y, Zhang H, Ye Y, Li J, Zhao L. HSP90α mediates sorafenib resistance in human hepatocellular carcinoma by necroptosis inhibition under hypoxia. Cancers (Basel) 2021;13:243. doi: 10.3390/cancers13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, Delrue I, Taminau J, Wiernicki B, De Groote P, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 52.Davola ME, Cormier O, Vito A, El-Sayes N, Collins S, Salem O, Revill S, Ask K, Wan Y, Mossman K. Oncolytic BHV-1 is sufficient to induce immunogenic cell death and synergizes with low-dose chemotherapy to dampen immunosuppressive T regulatory cells. Cancers (Basel) 2023;15:1295. doi: 10.3390/cancers15041295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Central Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel SJ, Protchenko O, Shakoury-Elizeh M, Baratz E, Jadhav S, Philpott CC. The iron chaperone and nucleic acid-binding activities of poly(rC)-binding protein 1 are separable and independently essential. Proc Natl Acad Sci USA. 2021;118:e2104666118. doi: 10.1073/pnas.2104666118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloomer SA, Brown KE. Hepcidin and iron metabolism in experimental liver injury. Am J Pathol. 2021;191:1165–1179. doi: 10.1016/j.ajpath.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye ZP, Peng XD, Li X, Huang Y, Zhu XY, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24:88–98. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 63.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, Schreiber SL. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Cao JY, Poddar A, Magtanong L, Lumb JH, Mileur TR, Reid MA, Dovey CM, Wang J, Locasale JW, Stone E, et al. A genome-wide haploid genetic screen identifies regulators of glutathione abundance and ferroptosis sensitivity. Cell Rep. 2019;26:1544–1556.e8. doi: 10.1016/j.celrep.2019.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao S, Yu J, He W, Huang Q, Zhao Y, Liang B, Zhang S, Wen Z, Dong S, Rao J, et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia. 2017;19:1022–1032. doi: 10.1016/j.neo.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Lu S, Wu LL, Yang L, Yang L, Wang J. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023;14:519. doi: 10.1038/s41419-023-06045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Central Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16:1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeitler L, Fiore A, Meyer C, Russier M, Zanella G, Suppmann S, Gargaro M, Sidhu SS, Seshagiri S, Ohnmacht C, et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife. 2021;10:e64806. doi: 10.7554/eLife.64806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 77.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 78.Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579–4590. doi: 10.1016/j.molcel.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R, Abbott DW, Xiao TS. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity. 2019;51:43–49.e4. doi: 10.1016/j.immuni.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 2017;38:261–271. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Fink SL, Cookson BT. Pillars article: Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]; J Immunol. 2019;202:1913–1926. [Google Scholar]
- 84.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 85.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 86.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 88.Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, Nie L, Chen Y, Wang YC, Liu C, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5:eaax7969. doi: 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erkes DA, Cai W, Sanchez IM, Purwin TJ, Rogers C, Field CO, Berger AC, Hartsough EJ, Rodeck U, Alnemri ES, Aplin AE. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y, Li D, Liu W, Zhang Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 96.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 97.Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22:412–422. doi: 10.1038/s41590-021-00869-6. [DOI] [PubMed] [Google Scholar]
- 98.Guo H, Callaway JB, Ting JP. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, Li Z, Wang Y, Zhao Q, Shao F, Ding J. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955.e20. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Loveless R, Bloomquist R, Teng Y. Pyroptosis at the forefront of anticancer immunity. J Exp Clin Canc Res. 2021;40:264. doi: 10.1186/s13046-021-02065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 102.Frank D, Vince JE. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight. 2019;4:e128834. doi: 10.1172/jci.insight.128834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: So similar, yet so different. Cell Death Differ. 2017;24:1172–1183. doi: 10.1038/cdd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Priem D, van Loo G, Bertrand MJM. A20 and cell death-driven inflammation. Trends Immunol. 2020;41:421–435. doi: 10.1016/j.it.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 106.Ye K, Chen Z, Xu Y. The double-edged functions of necroptosis. Cell Death Dis. 2023;14:163. doi: 10.1038/s41419-023-05691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mompeán M, Li W, Li J, Laage S, Siemer AB, Bozkurt G, Wu H, McDermott AE. The structure of the necrosome RIPK1-RIPK3 core, a human hetero-amyloid signaling complex. Cell. 2018;173:1244–1253.e10. doi: 10.1016/j.cell.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 110.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang W, Fan W, Guo J, Wang X. Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na+/H+ exchanger. Sci Signal. 2022;15:eabn5881. doi: 10.1126/scisignal.abn5881. [DOI] [PubMed] [Google Scholar]
- 113.Coriat R, Nicco C, Chéreau C, Mir O, Alexandre J, Ropert S, Weill B, Chaussade S, Goldwasser F, Batteux F. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 2012;11:2284–2293. doi: 10.1158/1535-7163.MCT-12-0093. [DOI] [PubMed] [Google Scholar]
- 114.Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 115.Huang CY, Chen LJ, Chen G, Chao TI, Wang CY. SHP-1/STAT3-signaling-axis-regulated coupling between BECN1 and SLC7A11 contributes to sorafenib-induced ferroptosis in hepatocellular carcinoma. Int J Mol Sci. 2022;23:11092. doi: 10.3390/ijms231911092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki S, Venkatesh D, Kanda H, Nakayama A, Hosokawa H, Lee E, Miki T, Stockwell BR, Yokote K, Tanaka T, Prives C. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res. 2022;82:3209–3222. doi: 10.1158/0008-5472.CAN-21-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y, Li L, Lan J, Cui Y, Rao X, Zhao J, Xing T, Ju G, Song G, Lou J, Liang J. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:11. doi: 10.1186/s12943-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang B, Bao W, Zhang S, Chen B, Zhou X, Zhao J, Shi Z, Zhang T, Chen Z, Wang L, et al. LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 2022;13:734. doi: 10.1038/s41419-022-05173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Q, Zheng W, Guan J, Liu H, Dan Y, Zhu L, Song Y, Zhou Y, Zhao X, Zhang Y, et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023;30:137–151. doi: 10.1038/s41418-022-01051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du J, Wan Z, Wang C, Lu F, Wei M, Wang D, Hao Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11:8185–8196. doi: 10.7150/thno.59121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Li X, Chen J, Zhang X, Guo J, Gu J, Mei C, Xiao Y, Peng C, Liu J, et al. Arsenic-loaded biomimetic iron oxide nanoparticles for enhanced ferroptosis-inducing therapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2023;15:6260–6273. doi: 10.1021/acsami.2c14962. [DOI] [PubMed] [Google Scholar]
- 122.Tian H, Zhao S, Nice EC, Huang C, He W, Zou B, Lin J. A cascaded copper-based nanocatalyst by modulating glutathione and cyclooxygenase-2 for hepatocellular carcinoma therapy. J Colloid Interface Sci. 2022;607:1516–1526. doi: 10.1016/j.jcis.2021.09.049. [DOI] [PubMed] [Google Scholar]
- 123.Su Y, Zhang Z, Lee LTO, Peng L, Lu L, He X, Zhang X. Amphiphilic dendrimer doping enhanced ph-sensitivity of liposomal vesicle for effective co-delivery toward synergistic ferroptosis-apoptosis therapy of hepatocellular carcinoma. Adv Healthc Mater. 2023;12:e2202663. doi: 10.1002/adhm.202202663. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, Zhang F, Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482–1505. doi: 10.1080/15548627.2019.1687985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 127.Shen M, Li Y, Wang Y, Shao J, Zhang F, Yin G, Chen A, Zhang Z, Zheng S. N6-methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021;47:102151. doi: 10.1016/j.redox.2021.102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301–310. doi: 10.1038/s41401-020-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Byun JK, Lee S, Kang GW, Lee YR, Park SY, Song IS, Yun JW, Lee J, Choi YK, Park KG. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:98. doi: 10.1186/s13046-022-02296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Byun JK. Tumor lactic acid: A potential target for cancer therapy. Arch Pharm Res. 2023;46:90–110. doi: 10.1007/s12272-023-01431-8. [DOI] [PubMed] [Google Scholar]
- 132.Zhang T, Sun L, Hao Y, Suo C, Shen S, Wei H, Ma W, Zhang P, Wang T, Gu X, et al. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1. Nat Cancer. 2022;3:75–89. doi: 10.1038/s43018-021-00299-1. [DOI] [PubMed] [Google Scholar]
- 133.Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, Cai K, Zhao Y, Luo Z. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 134.Yang Z, Su W, Wei X, Qu S, Zhao D, Zhou J, Wang Y, Guan Q, Qin C, Xiang J, et al. HIF-1α drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep. 2023;42:112945. doi: 10.1016/j.celrep.2023.112945. [DOI] [PubMed] [Google Scholar]
- 135.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sporn MB, Liby KT. NRF2 and cancer: The good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 138.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren X, Li Y, Zhou Y, Hu W, Yang C, Jing Q, Zhou C, Wang X, Hu J, Wang L, et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021;46:102122. doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K, Tang N. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12:426. doi: 10.1038/s41419-021-03718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, Martinez C, Su X, Rosato RR, Teng H, et al. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021;12:7333. doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu J, Dong Y, Ding L, Dong Y, Wu Z, Wang W, Shen M, Duan Y. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct Target Ther. 2019;4:28. doi: 10.1038/s41392-019-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shangguan F, Zhou H, Ma N, Wu S, Huang H, Jin G, Wu S, Hong W, Zhuang W, Xia H, Lan L. A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis. Front Cell Dev Biol. 2021;9:697832. doi: 10.3389/fcell.2021.697832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dai X, Sun F, Deng K, Lin G, Yin W, Chen H, Yang D, Liu K, Zhang Y, Huang L. Mallotucin D, a clerodane diterpenoid from croton crassifolius, suppresses HepG2 cell growth via inducing autophagic cell death and pyroptosis. Int J Mol Sci. 2022;23:14217. doi: 10.3390/ijms232214217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen Z, Zhou H, Li A, Wu T, Ji X, Guo L, Zhu X, Zhang D, He X. Metformin inhibits hepatocellular carcinoma development by inducing apoptosis and pyroptosis through regulating FOXO3. Aging (Albany NY) 2021;13:22120–22133. doi: 10.18632/aging.203464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Z, He M, Chen J, Li C, Zhang Q. Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol Lett. 2020;20:893–901. doi: 10.3892/ol.2020.11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kofahi HM, Taylor NGA, Hirasawa K, Grant MD, Russell RS. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep. 2016;6:37433. doi: 10.1038/srep37433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol Res. 2019;27:827–834. doi: 10.3727/096504018X15462920753012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Yang H, Sun M, He T, Liu Y, Yang X, Shi X, Liu X. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacol Rep. 2020;72:1370–1382. doi: 10.1007/s43440-020-00064-8. [DOI] [PubMed] [Google Scholar]
- 150.Wang F, Xu C, Li G, Lv P, Gu J. Incomplete radiofrequency ablation induced chemoresistance by up-regulating heat shock protein 70 in hepatocellular carcinoma. Exp Cell Res. 2021;409:112910. doi: 10.1016/j.yexcr.2021.112910. [DOI] [PubMed] [Google Scholar]
- 151.Seehawer M, Heinzmann F, D'Artista L, Harbig J, Roux PF, Hoenicke L, Dang H, Klotz S, Robinson L, Doré G, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schneider AT, Gautheron J, Feoktistova M, Roderburg C, Loosen SH, Roy S, Benz F, Schemmer P, Büchler MW, Nachbur U, et al. RIPK1 suppresses a TRAF2-dependent pathway to liver cancer. Cancer Cell. 2017;31:94–109. doi: 10.1016/j.ccell.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 153.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jomen W, Ohtake T, Akita T, Suto D, Yagi H, Osawa Y, Kohgo Y. Iron chelator deferasirox inhibits NF-κB activity in hepatoma cells and changes sorafenib-induced programmed cell deaths. Biomed Pharmacother. 2022;153:113363. doi: 10.1016/j.biopha.2022.113363. [DOI] [PubMed] [Google Scholar]
- 155.Harari-Steinfeld R, Gefen M, Simerzin A, Zorde-Khvalevsky E, Rivkin M, Ella E, Friehmann T, Gerlic M, Zucman-Rossi J, Caruso S, et al. The lncRNA H19-derived MicroRNA-675 promotes liver necroptosis by targeting FADD. Cancers (Basel) 2021;13:411. doi: 10.3390/cancers13030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng Y, Kong F, Liu S, Liu X, Pei D, Miao X. Membrane protein-chimeric liposome-mediated delivery of triptolide for targeted hepatocellular carcinoma therapy. Drug Deliv. 2021;28:2033–2043. doi: 10.1080/10717544.2021.1983072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mohammed S, Thadathil N, Selvarani R, Nicklas EH, Wang D, Miller BF, Richardson A, Deepa SS. Necroptosis contributes to chronic inflammation and fibrosis in aging liver. Aging Cell. 2021;20:e13512. doi: 10.1111/acel.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hammerich L, Tacke F. Eat more carrots? Dampening cell death in ethanol-induced liver fibrosis by β-carotene. Hepatobil Surg Nutr. 2013;2:248–251. doi: 10.3978/j.issn.2304-3881.2013.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao B, Lv X, Zhao X, Maimaitiaili S, Zhang Y, Su K, Yu H, Liu C, Qiao T. Tumor-promoting actions of HNRNP A1 in HCC are associated with cell cycle, mitochondrial dynamics, and necroptosis. Int J Mol Sci. 2022;23:10209. doi: 10.3390/ijms231810209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee SY, Kim S, Song Y, Kim N, No J, Kim KM, Seo HR. Sorbitol dehydrogenase induction of cancer cell necroptosis and macrophage polarization in the HCC microenvironment suppresses tumor progression. Cancer Lett. 2022;551:215960. doi: 10.1016/j.canlet.2022.215960. [DOI] [PubMed] [Google Scholar]
- 161.Lan W, Santofimia-Castaño P, Xia Y, Zhou Z, Huang C, Fraunhoffer N, Barea D, Cervello M, Giannitrapani L, Montalto G, et al. Targeting NUPR1 with the small compound ZZW-115 is an efficient strategy to treat hepatocellular carcinoma. Cancer Lett. 2020;486:8–17. doi: 10.1016/j.canlet.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 162.Tran DDH, Kessler C, Niehus SE, Mahnkopf M, Koch A, Tamura T. Myc target gene, long intergenic noncoding RNA, Linc00176 in hepatocellular carcinoma regulates cell cycle and cell survival by titrating tumor suppressor microRNAs. Oncogene. 2018;37:75–85. doi: 10.1038/onc.2017.312. [DOI] [PubMed] [Google Scholar]
- 163.Xiang YK, Peng FH, Guo YQ, Ge H, Cai SY, Fan LX, Peng YX, Wen H, Wang Q, Tao L. Connexin32 activates necroptosis through Src-mediated inhibition of caspase 8 in hepatocellular carcinoma. Cancer Sci. 2021;112:3507–3519. doi: 10.1111/cas.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xi G, Gao J, Wan B, Zhan P, Xu W, Lv T, Song Y. GSDMD is required for effector CD8+ T cell responses to lung cancer cells. Int Immunopharmacol. 2019;74:105713. doi: 10.1016/j.intimp.2019.105713. [DOI] [PubMed] [Google Scholar]
- 166.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T, et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 168.Snyder AG, Hubbard NW, Messmer MN, Kofman SB, Hagan CE, Orozco SL, Chiang K, Daniels BP, Baker D, Oberst A. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol. 2019;4:eaaw2004. doi: 10.1126/sciimmunol.aaw2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 170.Conche C, Finkelmeier F, Pešić M, Nicolas AM, Böttger TW, Kennel KB, Denk D, Ceteci F, Mohs K, Engel E, et al. Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut. 2023;72:1774–1782. doi: 10.1136/gutjnl-2022-327909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li S, Li F, Xu L, Liu X, Zhu X, Gao W, Shen X. TLR2 agonist promotes myeloid-derived suppressor cell polarization via Runx1 in hepatocellular carcinoma. Int Immunopharmacol. 2022;111:109168. doi: 10.1016/j.intimp.2022.109168. [DOI] [PubMed] [Google Scholar]
- 172.Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019;458:86–91. doi: 10.1016/j.canlet.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 173.Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, Pavelko KD, Li Y, O'Brien D, Wang C, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat rev immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 176.Hao X, Zheng Z, Liu H, Zhang Y, Kang J, Kong X, Rong D, Sun G, Sun G, Liu L, et al. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022;56:102463. doi: 10.1016/j.redox.2022.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen R, Li Q, Xu S, Ye C, Tian T, Jiang Q, Shan J, Ruan J. Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: From modulation to combination therapy targeting the microenvironment. Cancer Cell Int. 2022;22:73. doi: 10.1186/s12935-021-02435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li J, Yu J, Zhang T, Pu X, Li Y, Wu Z. Genomic analysis quantifies pyroptosis in the immune microenvironment of HBV-related hepatocellular carcinoma. Front immunol. 2022;13:932303. doi: 10.3389/fimmu.2022.932303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mohammed S, Nicklas EH, Thadathil N, Selvarani R, Royce GH, Kinter M, Richardson A, Deepa SS. Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radical Bio Med. 2021;164:315–328. doi: 10.1016/j.freeradbiomed.2020.12.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiang X, Deng W, Tao S, Tang Z, Chen Y, Tian M, Wang T, Tao C, Li Y, Fang Y, et al. A RIPK3-independent role of MLKL in suppressing parthanatos promotes immune evasion in hepatocellular carcinoma. Cell Discov. 2023;9:7. doi: 10.1038/s41421-022-00504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res. 2020;8:710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 183.Nicolè L, Sanavia T, Cappellesso R, Maffeis V, Akiba J, Kawahara A, Naito Y, Radu CM, Simioni P, Serafin D, et al. Necroptosis-driving genes RIPK1, RIPK3 and MLKL-p are associated with intratumoral CD3+ and CD8+ T cell density and predict prognosis in hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004031. doi: 10.1136/jitc-2021-004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pomlok K, Pata S, Kulaphisit M, Pangnuchar R, Wipasa J, Smith DR, Kasinrerk W, Lithanatudom P. An IgM monoclonal antibody against domain 1 of CD147 induces non-canonical RIPK-independent necroptosis in a cell type specific manner in hepatocellular carcinoma cells. Biochim Biophys Acta Mol Cell Res. 2022;1869:119295. doi: 10.1016/j.bbamcr.2022.119295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.