Figure 12. Consideration of Oral Anticoagulation for Device-Detected AHREs According to Patient Stroke Risk by CHA2DS2-VASc Score and Episode Duration.
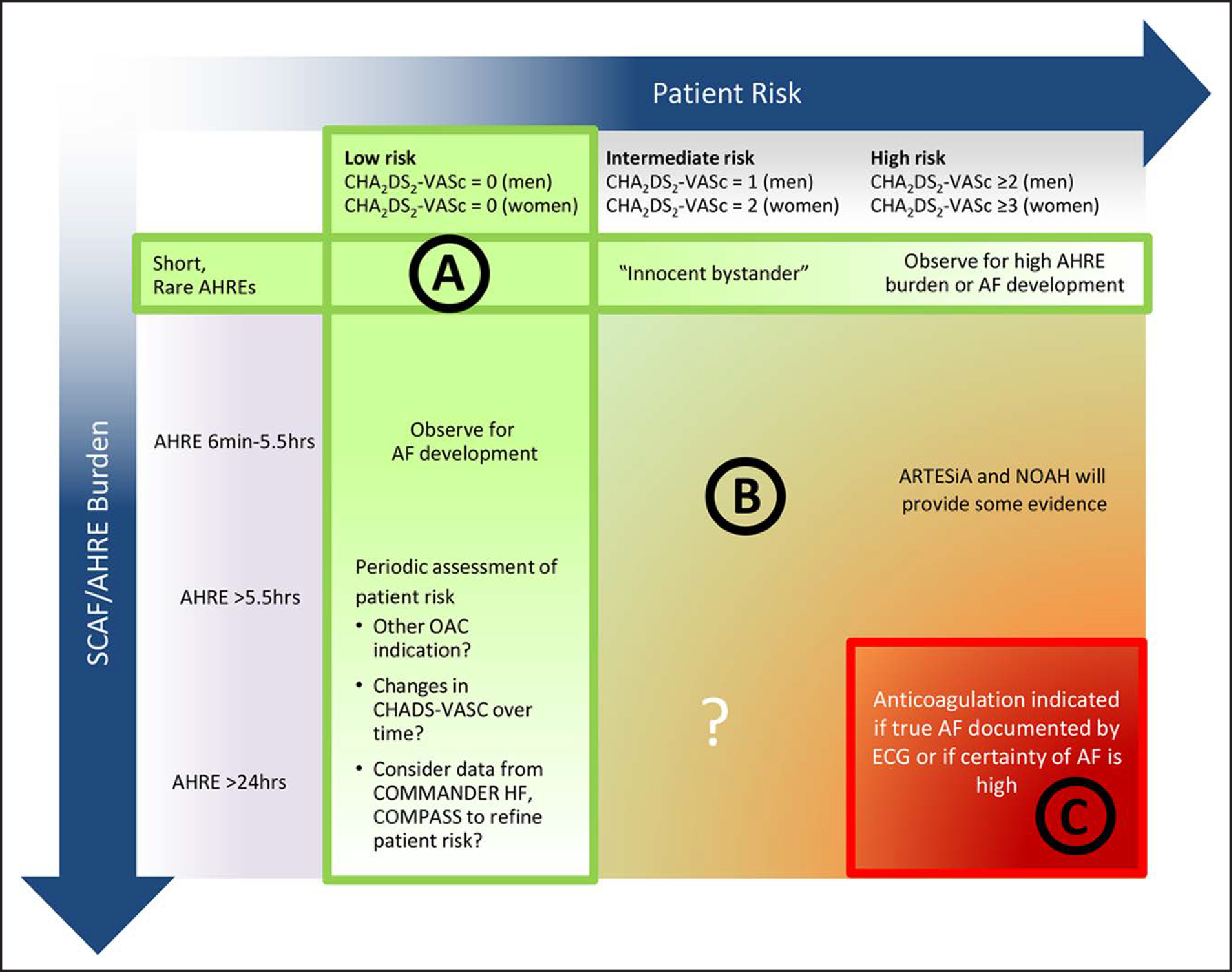
A potential approach to patients with SCAF could consider both patient risk (as gauged by the CHA2DS2-VASc score) and SCAF burden/duration. Circle A indicates patients at low risk or with short and infrequent AHREs do not require anticoagulation; Circle B, patients with intermediate risk and AHREs lasting >6 min to 24 h are an uncertain population but are currently under study in 2 prospective randomized controlled trials; and Circle C, patients at high risk with longer episodes could be considered reasonable candidates for anticoagulation, although the precise threshold for SCAF duration remains uncertain. Reproduced with permission from Noseworthy et al.13 Copyright 2019 American Heart Association, Inc. Modified from Freedman et al.14 Copyright 2017 Springer Nature Limited. AF indicates atrial fibrillation; AHRE, atrial high-rate episode; ARTESiA, Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Subclinical Atrial Fibrillation trial; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; ECG, electrocardiogram; NOAH, Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes Trial; OAC, oral anticoagulation; and SCAF, subclinical atrial fibrillation. Female sex is treated as a modifier in the computation of the CHA2DS2-VASc score.
