FIG 1.
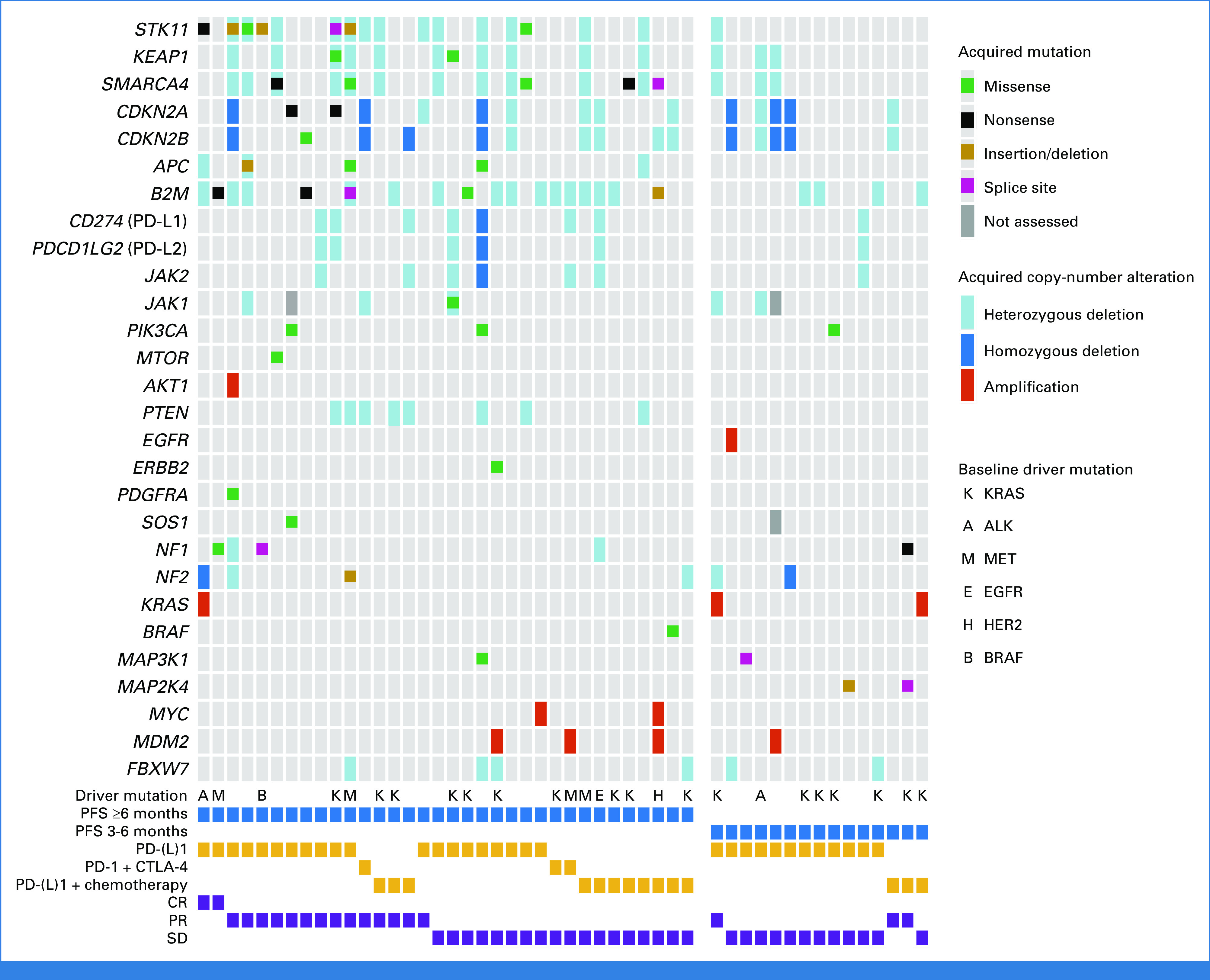
Summary of the genomic and immunophenotypic changes identified at the time of acquired resistance to PD-(L)1–based therapies in patients with NSCLC whose tumor underwent comprehensive genomic profiling at the DFCI. Only acquired genomic alterations in the post-ICI biopsy that were not present in the pre-ICI biopsy are displayed. Samples without acquired genomic changes at the time of resistance are not shown. Driver mutations shown in the oncoprint represent mutations identified at baseline, before the start of immunotherapy. Variants predicted to be benign or originating from clonal hematopoiesis of indeterminate potential are not shown. CR, complete response; CTLA-4, cytotoxic T-cell lymphocyte-4; DFCI, Dana-Farber Cancer Institute; ICI, immune checkpoint inhibitor; NSCLC, non–small-cell lung cancer; PFS, progression-free survival; PR, partial response; SD, stable disease.
