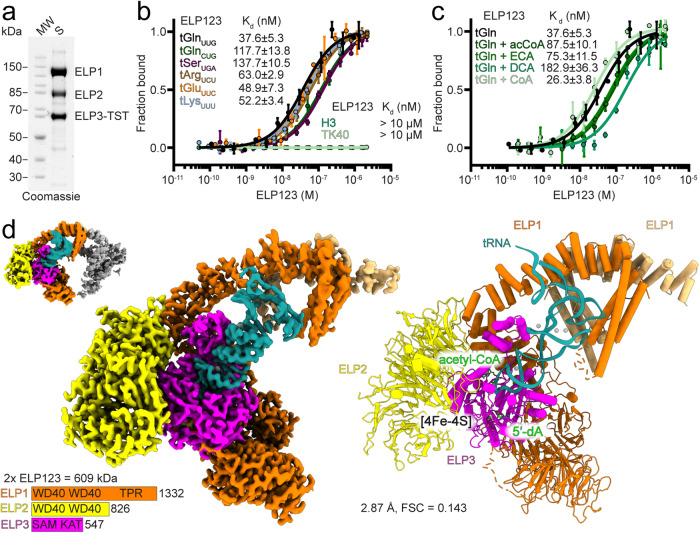
Fig. 1. Cryo-EM structure of human ELP123–tRNAGlnUUG–acetyl-CoA complex.
a SDS-PAGE analysis of purified ELP123 subcomplex. Predicted molecular weights (MW): ELP1 (148 kDa), ELP2 (93 kDa), ELP3 with Twin-Strep-Tag (TST; 65 kDa). b MST measurements with calculated dissociation constant (Kd) values for ELP123 bound to various tRNAs. n = 3 (independent experiments). Data are presented as mean values ± SEM. c MST measurements with Kd values for ELP123 bound to tRNAGlnUUG in the absence or presence of ligands (500 µM), including acetyl-CoA (acCoA), desulpho-Coenzym A (DCA) and S-Ethyl-Coenzym A (ECA). n = 3 (independent experiments). Data are presented as mean values ± SEM. d Cryo-EM density map (left) and atomic model (right) of ELP123 subcomplex bound to tRNA and acetyl-CoA (ELP1, orange; ELP1 second lobe, light orange; ELP2, yellow; ELP3, magenta and tRNAGlnUUG, deep teal) at a resolution of 2.87 Å. The two-lobe subclass density map is shown in the top left corner. Source data are provided as a Source Data file.