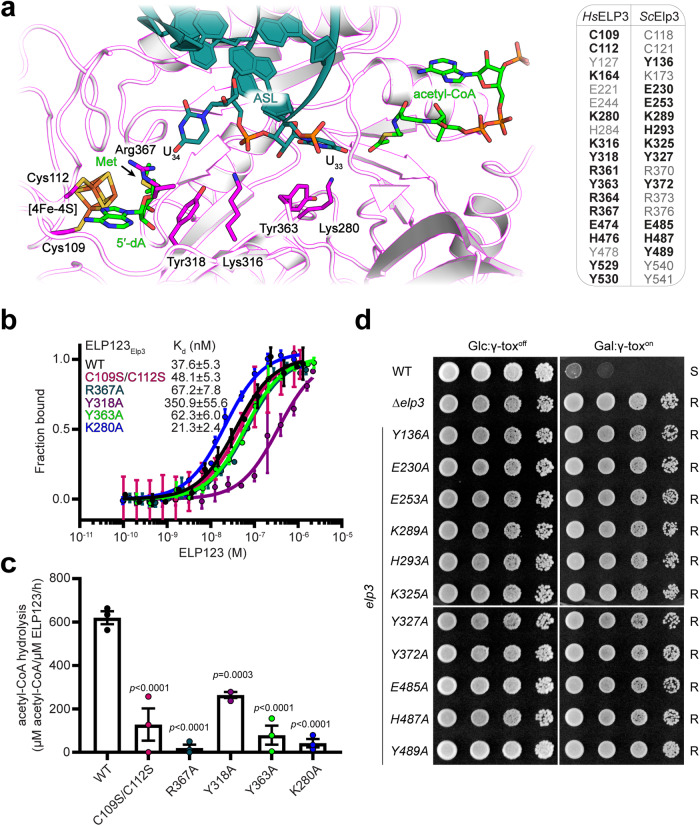
Fig. 5. Identification of a cluster of residues responsible for acetyl group transfer across ELP3 domains.
a Close-up view of the catalytic site of ELP3 with residues for Fe-S and SAM binding as well as acetyl-CoA transfer highlighted. The conserved residues in the catalytic site of ELP3, including human and yeast, are listed in the inlet. The tested residues in this study are highlighted in bold. b MST measurements with calculated Kd values for ELP123 bound to tRNAGlnUUG. n = 3 (independent experiments). Data are presented as mean values ± SEM. c Acetyl-CoA hydrolysis rates of ELP123 and ELP3 mutants in the presence of tRNAGlnUUG. n = 3 (independent experiments). Statistical analysis: one-way ANOVA with Dunnett’s multiple comparisons test. Statistically significant differences are indicated. Data are presented as mean values ± SEM. d Phenotype of yeast strains with Elp3 variants in response to galactose (Gal) induced γ-toxin expression. Sensitivity (‘S’) and resistance (‘R’) traits to growth inhibition by the Elongator-dependent tRNase toxin are appropriately labeled. Cell growth under glucose (Glc) conditions repressing toxin expression served as negative control. Source data are provided as a Source Data file.