Abstract
The purpose of this comprehensive literature review is to present the available evidence on the effects of methamphetamine on mental and oral health, as well as provide an overview of the most widely used medical and dental care strategies in the management of meth mouth. For this purpose, PubMed and Google Scholar electronic databases were searched for relevant articles, yielding 115 search results, which were further scrutinized for their relevance, leaving 55 for a detailed review. The analysis of the gathered data indicates that a comprehensive patient-centered approach that takes into consideration the physical, mental, and social aspects is crucial for mitigating the detrimental effects of increasing methamphetamine use.
Keywords: Methamphetamine, Literature Review, Crystal Meth, Meth Mouth
1. Introduction
There are four commonly misused drugs – cannabis, cocaine, opioids, and methamphetamine that cast long shadows on human health, each weaving a distinct web of adverse effects. Cannabis, while often viewed as less harmful, can damage the lungs and hinder brain development, and causes xerostomia which consequently increases the risk of dental caries. Cocaine, the seductive yet treacherous stimulant, unleashes a storm on the heart, raising blood pressure and risking strokes, causing bruxism and tooth wear. Opioids, pain relievers with high dependency rate, causing respiratory depression, organ damage, rampant caries and periodontal disease, and social unraveling. Finally, methamphetamine, the ruthless reaper, ravages the nervous system, leading to cognitive decline, dental problems, and skin sores (Teoh et al., 2019).
Methamphetamine (MA) is a potent central nervous system (CNS) stimulant that primarily acts by enhancing the release and inhibiting the reuptake of dopamine, norepinephrine, and serotonin in the brain (Chiu and Schenk, 2012, Fernández-Serrano et al., 2011, Gonzales et al., 2010, Jayanthi et al., 2021, Kim et al., 2020). This direct effect on these neurotransmitters increases their levels in the synapses, which in turn promotes CNS stimulation, leading to greater alertness, attention, and euphoria (Chiu and Schenk, 2012, Fernández-Serrano et al., 2011, Moszczynska and Callan, 2017, Shin et al., 2017). These neurotransmitters play important roles in regulating mood, appetite, and sleep, among other functions (O’Malley et al., 2022). Specifically, MA use can lead to changes in the expression of genes involved in dopamine signaling, synaptic plasticity, and stress responses (Xie & Miller, 2009). These changes are thought to contribute to the long-lasting effects of methamphetamine on the brain and behavior (Cruickshank and Dyer, 2009, Xie and Miller, 2009).
Methamphetamine also increases the activity of the hypothalamic − pituitary − adrenal (HPA) axis, as well as the release of corticotropin-releasing hormone (CRH) and cortisol (Chiu and Schenk, 2012, Scott et al., 2007, Shukla and Vincent, 2021). In addition to these impacts on the CNS, MA consumption promotes neuronal plasticity, thereby contributing to the development of addiction accompanied by changes in behavior (Zeng et al., 2023). As methamphetamine has the capacity to cross the blood − brain barrier (Zeng et al., 2023), upon entering the brain, it binds to and activates trace amine-associated receptor 1 (TAAR1)—a G-protein-coupled receptor that is expressed in several brain regions (Courtney & Ray, 2014). As a result of this activation, intracellular cyclic AMP (cAMP) levels increase, enabling the release of dopamine from presynaptic neurons (Courtney and Ray, 2014, Prakash et al., 2017).
Methamphetamine is a highly addictive stimulant that can be smoked, snorted, injected, or swallowed (Yang et al., 2018). This drug is often used in crystal form known as ‘meth’ or ‘ice’. While the number of addicts worldwide was estimated at 35 million in 2016, this figure is expected to become even higher given that meth is gradually replacing other illicit drugs such as cocaine and marijuana in many countries (Rommel et al., 2016a). Due to the availability and affordability of its components, MA is inexpensive to produce (Hamamoto & Rhodus, 2009), making it accessible to a wide variety of users. However, when meth is abused for a prolonged period, it can lead to addiction, as well as impairment in attention, executive functions, language/verbal fluency, and memory (Potvin et al., 2018, Rommel et al., 2016a). These psychological symptoms are often accompanied by cardiovascular problems, pulmonary arterial hypertension, renal failure, and meth mouth (Rommel et al., 2016a).
Chronic methamphetamine use wreaks havoc on both mental and physical health. Acutely, it unleashes a surge of dopamine, leading to euphoria, hyperactivity, and hypervigilance. However, this is followed by a profound “crash,” marked by depression, anxiety, and cravings (Rusyniak, 2011). Long-term, methamphetamine disrupts reward circuitry and leads to psychosis, including paranoia, hallucinations, and delusions (Volkow et al., 2001). Cognitive deficits, particularly in memory and decision-making, are also common (Sabrini et al., 2019). Systemically, methamphetamine wreaks havoc: cardiovascular complications like hypertension and cardiomyopathy are prevalent (Turdi et al., 2009). Neurotoxicity extends beyond the brain, damaging peripheral nerves and causing pain and neuropathy (Rusyniak, 2011). Organ systems like the kidneys, liver, and lungs are also susceptible to damage (Matsumoto et al., 2014). This devastating array of mental and systemic harm underscores the immense toll methamphetamine takes on individuals and healthcare systems.
While the adverse effects of meth are diverse, this literature review focuses specifically on extant studies related to its impact on users’ mental and oral health, and especially the medical management and prevention of meth mouth. This decision was guided by the limited evidence regarding the influence of methamphetamine (crystal meth) on oral health.
2. Materials and methods
2.1. Protocol and registration
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) protocol.
2.2. Focused question and search strategy
The focused question was determined according to the 2009 PICO strategy (Furlan et al., 2009). (1) Population: meth mouth, meth mouth AND oral health, methamphetamine abuse AND oral health, meth mouth prevention AND\ OR Meth mouth management, methamphetamine use management, methamphetamine addiction, methamphetamine psychological effects, methamphetamine mental health, and meth mouth AND addiction; (2) N/A. 3) Comparison: N/A. (4) Outcome: effect of meth. The focused questions of the present review was “Among the available studies on methamphetamine, what effect of the meth abuse based on?
2.3. Selection criteria
Inclusion criteria
-
1.
In vivo studies
-
2.
Case reports
-
3.
Literature review
-
4.
Methamphetamine user
Exclusion criteria
-
1.
In vitro studies
-
2.
Healthy patient
-
3.
Edentulous Patients
-
4.
Animal studies.
2.4. Search methods
Two independent authors (H. Q. and A.A) systematically searched the indexed literature in during January 2005–2023 period and updated it in December 2023. This literature review focused on studies related to methamphetamine use and its effects on oral health published in English language. For this purpose, PubMed and Google scholar electronic databases were searched using the terms meth mouth, meth mouth AND oral health, methamphetamine abuse AND oral health, meth mouth prevention AND\ OR Meth mouth management, methamphetamine use management, methamphetamine addiction, methamphetamine psychological effects, methamphetamine mental health, and meth mouth AND addiction.
Combinations of medical subject heading terms (MeSH) and non‐MeSH terms, along with Boolean operators, were utilized to perform the search. Relevant literature was also searched through Open Grey until December20223. A manual search of the available literature was also performed.
3. Results
After thorough assessment of the initial pool of 115 articles, only 55 were confirmed to meet the inclusion criteria and were retained for analysis, as in other cases the authors either relied on self-reports or the research design was subject to multiple limitations, including small sample size, quantities of methamphetamines used by the participants, the length of MA use, the route of administration, comorbid depressive symptoms, and alcohol use or cigarette smoking, which may adversely impact the results. (Fig. 1).
Fig. 1.
Flowchart of the selection of studies for methamphetamine.
4. Discussion
Meth is a potent psychostimulant that works by increasing dopamine, adrenaline, and noradrenaline levels in the body (Cossa et al., 2020, Yang et al., 2018). Hence, MA abusers experience hyperactivity, decreased appetite, increased alertness, insomnia, and hallucinations, and might develop psychotic as well as delusional disorders (Cossa et al., 2020, Glasner-Edwards and Mooney, 2014, Salo et al., 2011, Yang et al., 2018). Over time, addicts begin to manifest destructive psychological effects such as mood disturbances, agitation, paranoia, anxiety, and increased violence (Cossa et al., 2020, Darke et al., 2008), which can be exacerbated in some individuals that have previously suffered tragic events (Marshall & Werb, 2010). However, the influence of gender on the drug use history remains to be established, as some authors reported no difference in the time of use initiation between men and women (Simpson et al., 2016), while clinical evidence demonstrates that men use meth at higher rates than woman do (Daiwile et al., 2022). While men tend to have more complications that result in emergency department visits and more cardiovascular deaths, this is likely due to the fact that women usually realize the problem of meth addiction earlier than men do (Simpson et al., 2016). On the other hand, severity of psychological problems (both perceived and diagnosed) tends to be greater in women, who also tend to report greater childhood emotional or sexual trauma, suggesting that they use meth as a coping mechanism despite having more social support compared to men (Simpson et al., 2016).
According to studies focusing on the effect of MA abuse on oral health, it typically leads to extensive xerostomia, decreased saliva pH-values, reduced buffer capacity, leathery and rampant brown carious lesions which might extend to the free gingival margins, tooth erosion, dysgeusia, periodontal diseases, significant increase in plaque accumulation, calculus deposits, poor oral hygiene, and missing teeth (Clague et al., 2017, Heng et al., 2008, Mukherjee et al., 2018, Rommel et al., 2016a, Rommel et al., 2016b, Shetty et al., 2015, Turkyilmaz, 2010, Zokaee et al., 2022). The prevalence of dental condition that associated with using of MA among 301 user were dental appearance (28.6 percent, n = 86), broken or loose teeth (23.3 percent, n = 70) and tooth grinding or erosion (22.3 percent, n = 67) (Shetty et al., 2010). of Accordingly, the American Dental Association has proposed the term “meth mouth” to describe rampant caries frequently observed in methamphetamine users (Hamamoto and Rhodus, 2009, Rommel et al., 2015, Shaner et al., 2006), as they present with blackened, stained, rotting, and crumbling teeth at rates that exceed those in general population (Klasser & Epstein, 2005). In chronic MA users, the caries pattern is characteristic since it often occurs in the cervical region and progresses to the buccal smooth surface of the posterior teeth, as well as the interproximal areas of the anterior teeth (Wang et al., 2014). Available evidence also shows that persistent caries lesions and oral tissue inflammation can spread to other body parts and cause further lesions as well as diseases such as endocarditis (Rommel et al., 2015). These observations have led to the view that methamphetamine's impact on soft tissues and vascularization is what ultimately causes or initiates methamphetamine-induced osteonecrosis of the jaws (Pabst et al., 2017, Ristow et al., 2015, Rommel et al., 2015, Rustemeyer et al., 2014). Long-term MA usage has also been found to contribute to sinusitis and mucoceles of the maxillary sinus (Faucett et al., 2015). Consequently, even MA users who are otherwise in relatively good health suffer from dental or oral diseases and typically have considerably more missing teeth than non-users, especially if MA is smoked rather than taken intravenously (Shetty et al., 2010). Extant reach also shows that MA users have incredibly high levels of energy and neuro-muscular activity due to the sympathomimetic effects of this potent psychostimulant, which leads to parafunctional jaw activity, bruxism, muscle trismus, and lockjaw (Hamamoto and Rhodus, 2009, Mukherjee et al., 2018, Rhodus and Little, 2005).
4.1. Medical management of methamphetamine drug use
As methamphetamine is a highly addictive substance that can cause significant damage to the body and mind, medical management offered to users requires a comprehensive approach to address both the physical and mental health effects of the drug, which typically involves a combination of medication-assisted treatment, behavioral therapy, and support groups (Brown & DeFulio, 2020). Thus, even though several approaches are available, the most effective mode of MA addiction treatment will depend on the individual needs and circumstances (Brown & DeFulio, 2020). Some common treatment options include behavioral therapies, such as cognitive-behavioral therapy (CBT) and contingency management, which can help individuals identify and change negative patterns of thinking and behavior associated with drug abuse (Pabst et al., 2017). In clinical practice, medication-assisted treatments based on bupropion and naltrexone have also been shown to help reduce cravings and withdrawal symptoms (Skeer et al., 2022), while some addicts benefit from antidepressants, antipsychotics, and substitution/replacement therapy (Panenka et al., 2013). Nonetheless, residential treatment programs are always preferable, especially for individuals with severe MA addiction (Skeer et al., 2022), as they provide a structured environment, allowing them to focus on recovery and develop healthy coping skills (Shiao et al., 2021). Inpatient treatment can be particularly useful for individuals who have co-occurring mental health conditions or who require medical detoxification (Skeer et al., 2022). If this is not an option, outpatient treatment programs should be offered instead, as they allow individuals to receive treatment while continuing to live at home and attend work or school (Rhodus & Little, 2005). These treatments can be supplemented with support groups such as Narcotics Anonymous or SMART Recovery, as they enable participants to build a network of support during recovery (Rhodus and Little, 2005, Shiao et al., 2021). Still, it is important to acknowledge that recovery from MA addiction can be a long and challenging process. As a result, relapse is common, but with the right treatment and support, individuals can overcome addiction and lead fulfilling lives in recovery (Rhodus & Little, 2005). In order to sustain their abstinence, former MA addicts are also advised to make lifestyle changes such as improving diet and exercise habits, reducing stress, and avoiding triggers (Rhodus & Little, 2005).
In sum, medical management of methamphetamine drug use requires a comprehensive, individualized approach that addresses the physical, mental, social, and environmental factors that contribute to addiction (Petit et al., 2012). Treatment should be tailored to the specific needs of each person and should be provided by a multidisciplinary team of medical, mental health, and addiction specialists (Brown & DeFulio, 2020).
4.2. Dental management of methamphetamine drug use
Meth mouth is a term that describes an oral condition frequently found in MA users, and includes the characteristics such as xerostomia, extensive carious lesions, enamel erosion, bruxism, and muscle trismus (Pabst et al., 2017). While xerostomia is attributed to the reduction in the salivary flow due to MA use (Skeer et al., 2022), increased consumption of sugary beverages and food, combined with reduced oral hygiene (Shiao et al., 2021), would lead to severe dental problems. Consequently, oral management of meth mouth involves a combination of dental treatment and supportive care (Kamp et al., 2019, Tsui et al., 2020), which may involve a range of procedures, such as fillings, extractions, and periodontal surgery (Shetty et al., 2010). For individuals with extensive dental damage, full mouth reconstruction—using a combination of dental implants, multiple fixed dental prostheses, or removable or fixed complete overdentures to restore the appearance and function of the teeth—may be necessary (Shetty et al., 2010). This treatment is highly beneficial, as it improves MA users’ quality of life and acceptance in society (Alqarni et al., 2022). However, these individuals will also require supportive care to manage meth mouth symptoms and prevent further damage (Shetty et al., 2010). This should include regular dental check-ups to monitor the progress of the disease and prevent further damage, oral hygiene education to promote healthy brushing and flossing habits, and nutritional counseling to address malnutrition and promote healthy eating habits (Cossa et al., 2020). It is important to note that dental treatment for meth mouth can be complex, and it may take several visits to address the full extent of the damage (Shetty et al., 2010, Smit and Naidoo, 2015). In some cases, dental treatment may need to be coordinated with addiction treatment to ensure that individuals are addressing both the physical and mental health effects of methamphetamine use (Cossa et al., 2020).
In sum, oral management of meth mouth involves a comprehensive approach that addresses both the dental problems associated with MA use and the underlying addiction (Degenhardt et al., 2017), as this reduces the likelihood of further damage to the teeth and gums (Cossa et al., 2020). Nonetheless, further research is needed to understand the risk factors that lead to MA addition, undermine recovery, and increase the likelihood of problems discussed in this work (Gonzales et al., 2009, Marshall and Werb, 2010).
5. Conclusion
Methamphetamine is a highly addictive and dangerous drug with severe medical and dental health consequences. It is thus important to be aware of the risks of meth use, especially given the drug's increasing availability and affordability. Methamphetamine can cause extensive oral health problems, including bruxism and excessive neuromuscular activity, leading to parafunctional temporomandibular function and drastically decreased saliva production. This combination of factors can lead to a unique and rapid pattern of dental caries, compounded by poor hygiene habits.
Therefore, it is important to manage all these factors from both medical and dental perspective. Medical management of meth drug use requires a comprehensive, person-centered approach that addresses the physical, mental, and social factors of addiction. This approach should involve a multidisciplinary team of healthcare providers. Oral health management is also important to address the underlying dental problems and restore oral health (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6). In summary, meth use is strongly discouraged due to its addictive nature and devastating impact on users' health and well-being.
Fig. 2.
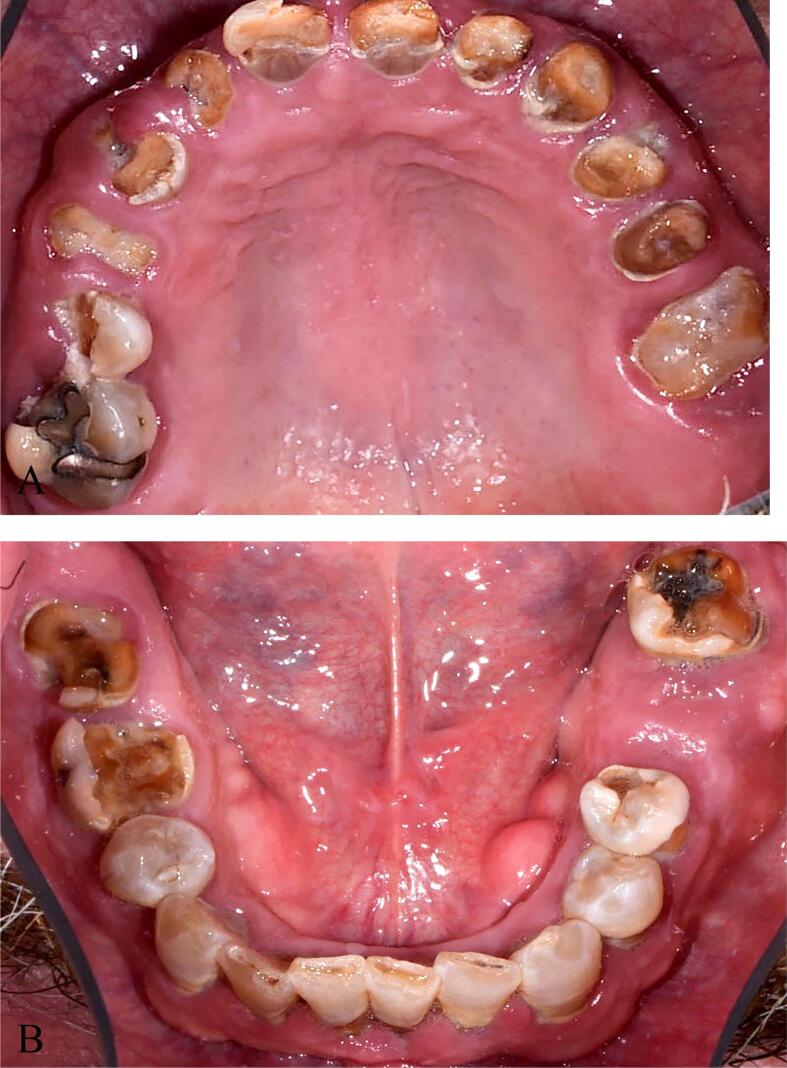
Pre-treatment intraoral occlusal view of maxillary and mandibular arches presenting “meth mouth”. Remaining roots, leathery and rampant brown carious lesions, erosion, plaque accumulation, calculus, missing tooth and periodontal inflammation are shown. A, Maxilla B, mandible.
Fig. 3.
Post-treatment intraoral occlusal view of maxillary and mandibular arches presenting the definitive prosthesis (metal ceramic screw-retained implant-supported fixed dental prosthesis). A, Maxilla. B, Mandible.
Fig. 4.
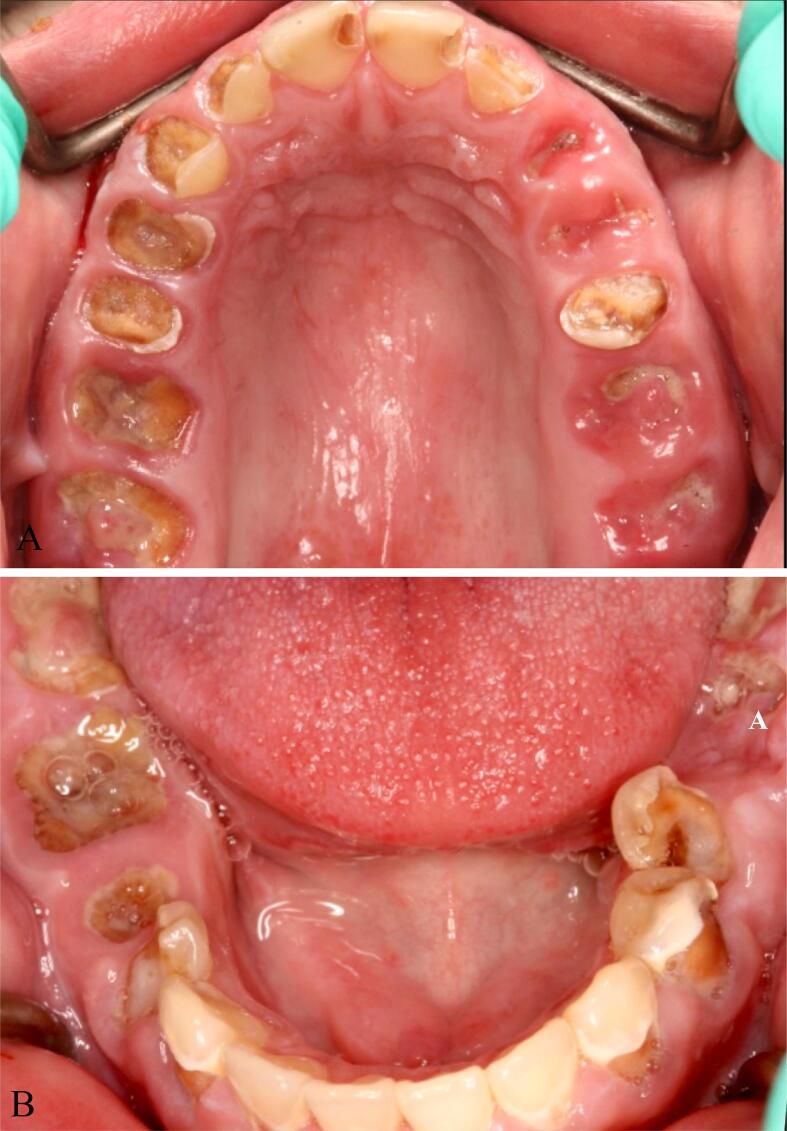
Pre-treatment intraoral occlusal view of maxillary and mandibular arches of a crystal meth user. Residual roots, multiple carious lesions, calculus deposits, and poor oral hygiene are shown. A, Maxilla B, mandible.
Fig. 5.
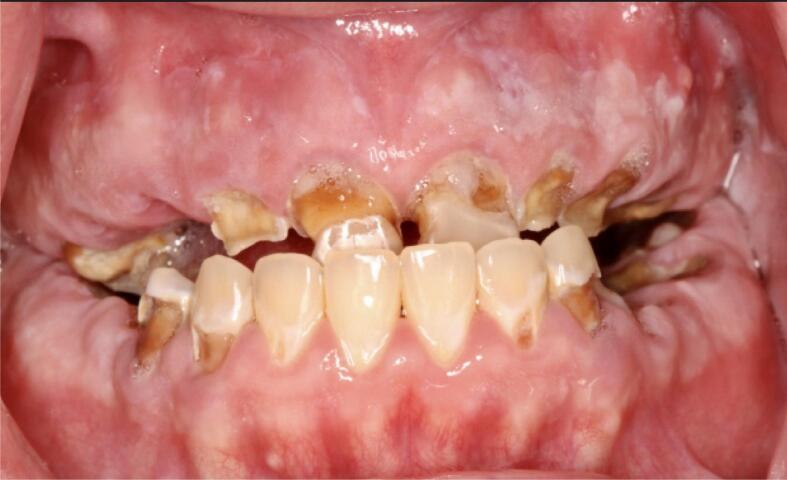
Pre-treatment intraoral frontal view of a crystal meth user. Loss of VDO, reverse articulation, residual roots, and rampant brown carious lesions are shown.
Fig. 6.
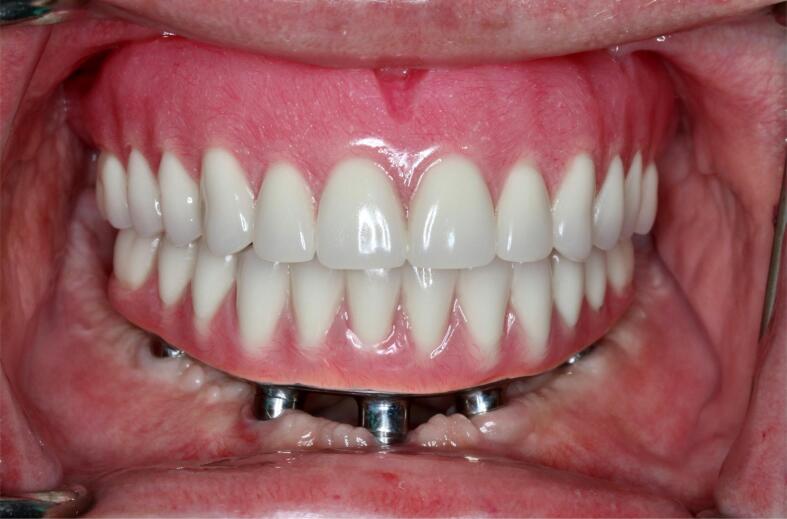
Post-treatment intraoral frontal view presenting the definitive prosthesis (mandibular implant-supported fixed dental prosthesis) and (maxillary removable complete denture).
CRediT authorship contribution statement
Hatem Alqarni: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Adhwaa Aldghim: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Rose Alkahtani: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Nasser Alshahrani: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Majed S. Altoman: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Visualization, Writing – original draft, Supervision. Mohammed A. Alfaifi: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Visualization,: Writing – original draft, Supervision. Mohammad Helmi: Methodology, Formal analysis, Writing – review & editing, Writing – original draft, Visualization, Supervision. Abdulaziz A. Alzaid: Methodology, Formal analysis, Writing – review & editing, Writing – original draft, Visualization, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alqarni H., Alsaloum M., Alzaid A. Prosthetic rehabilitation of meth mouth with implant-supported fixed dental prostheses: a clinical report. J. Prosthet. Dent. 2022;128(6):1140–1144. doi: 10.1016/j.prosdent.2021.02.036. [DOI] [PubMed] [Google Scholar]
- Brown H.D., DeFulio A. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alcohol Depend. 2020;216 doi: 10.1016/j.drugalcdep.2020.108307. [DOI] [PubMed] [Google Scholar]
- Chiu V., Schenk J. Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Curr. Drug Abuse Rev. 2012;5:227–242. doi: 10.2174/1874473711205030227. [DOI] [PubMed] [Google Scholar]
- Clague J., Belin T.R., Shetty V. Mechanisms underlying methamphetamine-related dental disease. J. Am. Dent. Assoc. (1939) 2017;148(6):377–386. doi: 10.1016/j.adaj.2017.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossa F., Piastra A., Sarrion-Pérez M.G., Bagán L. Oral manifestations in drug users: a review. J. Clin. Exp. Dent. 2020;12(2):e193–e200. doi: 10.4317/jced.55928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K.E., Ray L.A. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank C.C., Dyer K.R. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Daiwile A.P., Jayanthi S., Cadet J.L. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: potential therapeutic impacts. Neurosci. Biobehav. Rev. 2022;137 doi: 10.1016/j.neubiorev.2022.104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S., Kaye S., Mcketin R., Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27(3):253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Sara G., McKetin R., Roxburgh A., Dobbins T., Farrell M., Burns L., Hall W.D. Crystalline methamphetamine use and methamphetamine-related harms in Australia. Drug Alcohol Rev. 2017;36(2):160–170. doi: 10.1111/dar.12426. [DOI] [PubMed] [Google Scholar]
- Faucett E.A., Marsh K.M., Farshad K., Erman A.B., Chiu A.G. Maxillary sinus manifestations of methamphetamine abuse. Allergy & Rhinology (Providence, R.I.) 2015;6(1):76–79. doi: 10.2500/ar.2015.6.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano M.J., Pérez-García M., Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci. Biobehav. Rev. 2011;35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Furlan A.D., Pennick V., Bombardier C., van Tulder M., Editorial Board C.B.R.G. 2009 updated method guidelines for systematic reviews in the cochrane back review group. Spine. 2009;34:13. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S., Mooney L.J. Methamphetamine psychosis: epidemiology and management. CNS Drugs. 2014;28(12):1115–1126. doi: 10.1007/s40263-014-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R., Ang A., Marinelli-Casey P., Glik D.C., Iguchi M.Y., Rawson R.A. Health-related quality of life trajectories of methamphetamine-dependent individuals as a function of treatment completion and continued care over a 1-year period. J. Subst. Abuse Treat. 2009;37(4):353–361. doi: 10.1016/j.jsat.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Gonzales R., Mooney L., Rawson R.A. The methamphetamine problem in the United States. Annu. Rev. Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto D.T., Rhodus N.L. Methamphetamine abuse and dentistry. Oral Dis. 2009;15(1):27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Heng C., Badner V., Schiop L. Meth mouth. N. Y. State Dent. J. 2008;74:50–51. [PubMed] [Google Scholar]
- Jayanthi S., Daiwile A.P., Cadet J.L. Neurotoxicity of methamphetamine: main effects and mechanisms. Exp. Neurol. 2021;344 doi: 10.1016/j.expneurol.2021.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F., Proebstl L., Hager L., Schreiber A., Riebschläger M., Neumann S., Straif M., Schacht-Jablonowsky M., Manz K., Soyka M., Koller G. Effectiveness of methamphetamine abuse treatment: predictors of treatment completion and comparison of two residential treatment programs. Drug Alcohol Depend. 2019;201:8–15. doi: 10.1016/j.drugalcdep.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Kim B., Yun J., Park B. Methamphetamine-induced neuronal damage: neurotoxicity and neuroinflammation. Biomol. Ther. 2020;28(5):381–388. doi: 10.4062/biomolther.2020.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasser G.D., Epstein J. Methamphetamine and its impact on dental care. J. Can. Dent. Assoc. 2005;71(10):759–762. [PubMed] [Google Scholar]
- Marshall B.D.L., Werb D. Health outcomes associated with methamphetamine use among young people: a systematic review. Addiction. 2010;105(6):991–1002. doi: 10.1111/j.1360-0443.2010.02932.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto R.R., Seminerio M.J., Turner R.C., Robson M.J., Nguyen L., Miller D.B., O’Callaghan J.P. Methamphetamine-induced toxicity: an updated review on issues related to hyperthermia. Pharmacol. Ther. 2014;144(1):28–40. doi: 10.1016/j.pharmthera.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczynska A., Callan S.P. Molecular, behavioral, and physiological consequences of methamphetamine neurotoxicity: implications for treatment. J. Pharmacol. Exp. Ther. 2017;362(3):474–488. doi: 10.1124/jpet.116.238501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Dye B.A., Clague J., Belin T.R., Shetty V. Methamphetamine use and oral health-related quality of life. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehab. 2018;27(12):3179–3190. doi: 10.1007/s11136-018-1957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley K.Y., Hart C.L., Casey S., Downey L.A. Methamphetamine, amphetamine, and aggression in humans: a systematic review of drug administration studies. Neurosci. Biobehav. Rev. 2022;141 doi: 10.1016/j.neubiorev.2022.104805. [DOI] [PubMed] [Google Scholar]
- Pabst A., Castillo-Duque J.C., Mayer A., Klinghuber M., Werkmeister R. Meth mouth-a growing epidemic in dentistry? Dent. J. 2017;5(4):29. doi: 10.3390/dj5040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenka W.J., Procyshyn R.M., Lecomte T., MacEwan G.W., Flynn S.W., Honer W.G., Barr A.M. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Petit A., Karila L., Chalmin F. Methamphetamine addiction: a review of the literature. J. Addict. Res. Ther. 2012;01 doi: 10.4172/2155-6105.S1-006. [DOI] [Google Scholar]
- Potvin S., Pelletier J., Grot S., Hébert C., Barr A.M., Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict. Behav. 2018;80:154–160. doi: 10.1016/j.addbeh.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Prakash M.D., Tangalakis K., Antonipillai J., Stojanovska L., Nurgali K., Apostolopoulos V. Methamphetamine: effects on the brain, gut and immune system. Pharmacol. Res. 2017;120:60–67. doi: 10.1016/j.phrs.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Rhodus N., Little J. Methamphetamine abuse and “meth mouth”. Pa. Dent. J. 2005;75:19–29. [PubMed] [Google Scholar]
- Ristow O., Otto S., Troeltzsch M., Hohlweg-Majert B., Pautke C. Treatment perspectives for medication-related osteonecrosis of the jaw (MRONJ) J. Cranio-Maxillofac. Surg. 2015;43(2):290–293. doi: 10.1016/j.jcms.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Rommel N., Rohleder N.H., Wagenpfeil S., Haertel-Petri R., Kesting M.R. Evaluation of methamphetamine-associated socioeconomic status and addictive behaviors, and their impact on oral health. Addict. Behav. 2015;50:182–187. doi: 10.1016/j.addbeh.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Rommel N., Rohleder N.H., Koerdt S., Wagenpfeil S., Härtel-Petri R., Wolff K.-D., Kesting M.R. Sympathomimetic effects of chronic methamphetamine abuse on oral health: a cross-sectional study. BMC Oral Health. 2016;16(1):59. doi: 10.1186/s12903-016-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel N., Rohleder N.H., Wagenpfeil S., Härtel-Petri R., Jacob F., Wolff K.-D., Kesting M.R. The impact of the new scene drug “crystal meth” on oral health: a case–control study. Clin. Oral Invest. 2016;20(3):469–475. doi: 10.1007/s00784-015-1527-z. [DOI] [PubMed] [Google Scholar]
- Rustemeyer J., Melenberg A., Junker K., Sari-Rieger A. Osteonecrosis of the maxilla related to long-standing methamphetamine abuse: a possible new aspect in the etiology of osteonecrosis of the jaw. Oral Maxillofac. Surg. 2014;18(2):237–241. doi: 10.1007/s10006-014-0449-2. [DOI] [PubMed] [Google Scholar]
- Rusyniak D.E. Neurologic Manifestations of Chronic Methamphetamine Abuse. Neurol. Clin. 2011;29(3):641–655. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrini S., Wang G.Y., Lin J.C., Ian J.K., Curley L.E. Methamphetamine use and cognitive function: a systematic review of neuroimaging research. Drug Alcohol Depend. 2019;194:75–87. doi: 10.1016/j.drugalcdep.2018.08.041. [DOI] [PubMed] [Google Scholar]
- Salo R., Flower K., Kielstein A., Leamon M.H., Nordahl T.E., Galloway G.P. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186(2–3):356–361. doi: 10.1016/j.psychres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.C., Woods S.P., Matt G.E., Meyer R.A., Heaton R.K., Atkinson J.H., Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shaner J.W., Kimmes N., Saini T., Edwards P. “Meth Mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDS. 2006;20(3):146–150. doi: 10.1089/apc.2006.20.146. [DOI] [PubMed] [Google Scholar]
- Shetty V., Mooney L.J., Zigler C.M., Belin T.R., Murphy D., Rawson R. The relationship between methamphetamine use and increased dental disease. J. Am. Dent. Assoc. (1939) 2010;141(3):307–318. doi: 10.14219/jada.archive.2010.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty V., Harrell L., Murphy D.A., Vitero S., Gutierrez A., Belin T.R., Dye B.A., Spolsky V.W. Dental disease patterns in methamphetamine users: findings in a large urban sample. J. Am. Dent. Assoc. (1939) 2015;146(12):875–885. doi: 10.1016/j.adaj.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao Y.-H., Chen Y.-C., Yeh Y.-C., Huang T.-H. Positive effects of laser acupuncture in methamphetamine users undergoing group cognitive behavioral therapy: a pilot study. Evid. Based Complement. Alternat. Med. 2021;2021:5514873. doi: 10.1155/2021/5514873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E.-J., Dang D.-K., Tran T.-V., Tran H.-Q., Jeong J.H., Nah S.-Y., Jang C.-G., Yamada K., Nabeshima T., Kim H.-C. Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch. Pharm. Res. 2017;40(4):403–428. doi: 10.1007/s12272-017-0897-y. [DOI] [PubMed] [Google Scholar]
- Shukla M., Vincent B. Methamphetamine abuse disturbs the dopaminergic system to impair hippocampal-based learning and memory: An overview of animal and human investigations. Neurosci. Biobehav. Rev. 2021;131:541–559. doi: 10.1016/j.neubiorev.2021.09.016. [DOI] [PubMed] [Google Scholar]
- Simpson J.L., Grant K.M., Daly P.M., Kelley S.G., Carlo G., Bevins R.A. Psychological burden and gender differences in methamphetamine-dependent individuals in treatment. J. Psychoact. Drugs. 2016;48(4):261–269. doi: 10.1080/02791072.2016.1213470. [DOI] [PubMed] [Google Scholar]
- Skeer M.R., Landy D.M., Ryan E.C., Lee-Bravatti M., Boyadjian T., Towers J. “(Meth) Will Hurt You and Hurt Your Teeth”: teen, parent, and dental practitioner perspectives on implementing crystal meth use prevention messaging in the dental office setting. Int. J. Dent. 2022;2022:6933091. doi: 10.1155/2022/6933091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit D.A., Naidoo S. Oral health effects, brushing habits and management of methamphetamine users for the general dental practitioner. Br. Dent. J. 2015;218(9):531–536. doi: 10.1038/sj.bdj.2015.341. [DOI] [PubMed] [Google Scholar]
- Teoh L., Moses G., McCullough M. Oral manifestations of illicit drug use. Aust. Dent. J. 2019;64(3):213–222. doi: 10.1111/adj.12709. [DOI] [PubMed] [Google Scholar]
- Tsui J.I., Mayfield J., Speaker E.C., Yakup S., Ries R., Funai H., Leroux B.G., Merrill J.O. Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. J. Substance Abuse Treat. 2020;109(October):80–85. doi: 10.1016/j.jsat.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Turdi S., Schamber R.M., Roe N.D., Chew H.G., Culver B., Ren J. Acute methamphetamine exposure inhibits cardiac contractile function. Toxicol. Lett. 2009;189(2):152–158. doi: 10.1016/j.toxlet.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkyilmaz I. Oral manifestations of “meth mouth”: a case report. J Contemp Dent Pract. 2010;11(1):E073–E080. [PubMed] [Google Scholar]
- Volkow N.D., Chang L., Wang G.-J., Fowler J.S., Franceschi D., Sedler M., Gatley S.J., Miller E., Hitzemann R., Ding Y.-S., Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen X., Zheng L., Guo L., Li X., Shen S. Comprehensive dental treatment for “meth mouth”: a case report and literature review. J. Formos. Med. Assoc. 2014;113(11):867–871. doi: 10.1016/j.jfma.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Xie Z., Miller G.M. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J. Pharmacol. Exp. Ther. 2009;330(1):316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang Y., Li Q., Zhong Y., Chen L., Du Y., He J., Liao L., Xiong K., Yi C., Yan J. The main molecular mechanisms underlying methamphetamine- induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci. 2018;11 doi: 10.3389/fnmol.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R., Pu H., Zhang X., Yao M., Sun Q. Methamphetamine: mechanism of action and Chinese herbal medicine treatment for its addiction. Chin. J. Integr. Med. 2023;29(7):665–672. doi: 10.1007/s11655-023-3635-y. [DOI] [PubMed] [Google Scholar]
- Zokaee H., Fathi S., Golalipour H., Mirzaei F. Effects of methamphetamine withdrawal on the volume and pH of stimulated saliva. Journal of Dentistry (Shiraz, Iran) 2022;23(2):80–85. doi: 10.30476/DENTJODS.2021.87248.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]