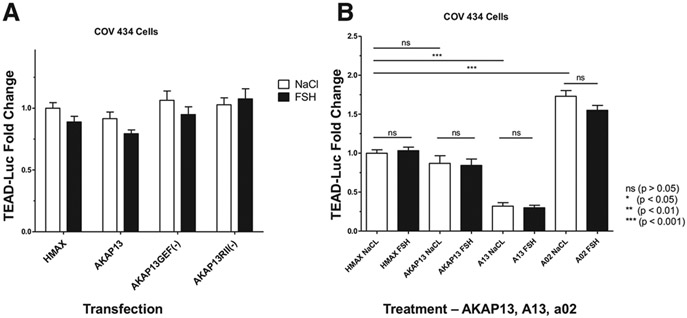
FIGURE 3.
A-kinase anchoring protein 13 manipulation. (A) No significant changes in TEAD-TEAD-luc expression with AKAP13 overexpression and AKAP13 mutants and with and without FSH treatment. The GEF-AKAP13 mutant, GEF(-), has a single point mutation: tyrosine to phenylalanine at position 2153 (Y2153F), which eliminates the GEF activity of AKAP13 and downstream Rho activity. The protein kinase A regulatory subunit II (RII)-AKAP13 mutant, RII(-), has 2 point mutations: alanine to proline at position 1251 and isoleucine to proline at position 1260 (A1251P/I1260P). This mutant disrupts binding of the RII regulatory subunit to AKAP13, thus interfering with activation of protein kinase A by AKAP13. The x-axis indicates the various vectors transfected into the COV434 cells, and the y-axis shows TEAD-luc in RLU fold change. White bars are cells treated with 0.9% NaCl, and black bars are cells treated with 1 IU of FSH (GONAL-f; Merck Serono, Darmstadt, Germany). No significant differences were found for the overall 2-way ANOVA or for each of the paired transfection comparisons between NaCl and FSH treatment. The results showing that there was no effect after treating with FSH support the idea that TEAD in the Hippo pathway is part of the gonadotropin-independent portion of folliculogenesis. There were also no differences between empty vector HMax-AKAP13 (HMax), AKAP13 overexpression, and GEF(-) or RII(-) mutant transfected cells treated with NaCl, which may indicate that the endogenous AKAP13 is already at its maximum use. Samples were analyzed with 3 biologic replicates and 3 technical replicates each. Error bars = standard error of the mean. Statistical analysis was performed using a permutation 2-way ANOVA followed by a post hoc quantile 1-way ANOVA to compare each pair of experimental groups. Data are presented with false discovery rate-adjusted P values. *P<.05, **P<.01, and ***P<.001. (B) The effect of A13 and A02 on TEAD-luc with AKAP13 overexpression plus either NaCl or FSH treatment. The x-axis indicates the vector transfected (HMax or AKAP13 overexpression) or treatment with the AKAP13 inhibitor (A13) or AKAP13 activator (A02) in COV434 cells. The y-axis shows TEAD-luc in RLU fold change. White bars are cells treated with 0.9% NaCl, and black bars are cells treated with 1 IU of FSH (GONAL-f; Merck Serono). Addition of AKAP13 did not augment TEAD-luc reporter activity, possibly due to high levels of endogenous AKAP13 in COV434 cells. Treatment with A13 reduced TEAD-luc activity by 68% (P <.0001), and treatment with A02 increased TEAD-luc activity by 73% (P <.0001). Samples were analyzed with 3 biologic replicates and 3 technical replicates each. Error bars = standard error of the mean. Statistical analysis was performed using a permutation 2-way ANOVA followed by a post hoc quantile 1-way ANOVA to compare each pair of experimental groups where the permutation 2-way ANOVA results were statistically significant. Data are presented with false discovery rate-adjusted P values. *P<.05, **P<.01, and ***P<.001. . AKAP13 = A-kinase anchoring protein 13; ECM = extracellular matrix; FSH = follicle-stimulating hormone; GEF = guanine nucleotide exchange factor; GTP = guanosine-5′-triphosphate; RLU = relative luciferase unit; TEAD = Tea domain family; TEAD-luc = TEAD luciferase.