Abstract
Introduction
Venous thromboembolism (VTE) is a significant contributor to morbidity and mortality among patients recovering from aneurysmal subarachnoid hemorrhage (aSAH). Prophylactic heparin reduces the risk of VTE, but the optimal timing for its initiation among aSAH patients remains unclear.
Objective
To conduct a retrospective study assessing risk factors for VTE and optimal timing of chemoprophylaxis in patients treated for aSAH.
Methods
From 2016 to 2020, 194 adult patients were treated for aSAH at our institution. Patient demographics, clinical diagnoses, complications, pharmacologic interventions, and outcomes were recorded. Risk factors for symptomatic VTE (sVTE) were analyzed via Chi-squared, univariate, and multivariate regression.
Results
In total 33 patients presented with sVTE (25 DVT, 14 PE). Patients with sVTE had longer hospital stays (p<0.01) and worse outcomes at one-month (p<0.01) and three-month follow-up (p= 0.02). Univariate predictors of sVTE included male sex (p=0.03), Hunt Hess score (p=0.01), Glasgow Coma scale (p=0.02), intracranial hemorrhage (p=0.03), hydrocephalus requiring external ventricular drain (EVD) placement (p<0.01), and mechanical ventilation (p<0.01). Only hydrocephalus requiring EVD (p=0.01) and ventilator use (p=0.02) remained significant upon multivariate analysis. Patients with delayed heparin introduction were significantly more likely to sustain sVTE on univariate analysis (p=0.02) with a trend-level significance on multivariate analysis (p=0.07).
Conclusions
Patients with aSAH are more likely to develop sVTE following use of perioperative EVD or mechanical ventilation. sVTE leads to longer hospital stays and worse outcomes among patients treated for aSAH. Delayed heparin initiation increases the risk of sVTE. Our results may help guide surgical decision-making during recovery from aSAH and improve VTE-related postoperative outcomes.
Keywords: Aneurysmal subarachnoid hemorrhage, deep vein thrombosis, pulmonary embolism, heparin, prophylaxis, timing
Introduction:
Aneurysmal subarachnoid hemorrhage (aSAH) is a complex disorder with a 35% mortality and substantial lasting disabilities.1 Nearly 25% of patients die before reaching medical attention while survivors face the risk of secondary disease including aneurysm re-rupture and vasospasm.2 Among these complications are deep vein thrombosis (DVT) and pulmonary embolism (PE), which have been shown to emerge in roughly 20% of patients, typically within the first two weeks post-hemorrhage.3, 4 These venous thromboembolisms (VTE) significantly increase morbidity and mortality among neurosurgical patients.5
Several risk factors associated with VTE have been identified in patients after aSAH, including male sex, coagulopathies, neurologic disorders, and obesity.6 Identifying additional risk factors can aid clinicians in determining which patients may most benefit from aggressive preventative therapies. Previous work has also shown that heparin prophylaxis in neurosurgical patients significantly reduces the risk of VTE.7, 8 However, early heparinization following surgery, especially in a patient suffering from an acute bleed, may result in hemorrhagic complications.9 Thus, it remains unclear when is optimal to initiate heparin in patients recovering from aSAH.
We sought to determine the factors impacting VTE risk among patients treated for aSAH. In addition, we aimed to assess the optimal timing of heparin debut following treatment for aSAH to minimize VTE while limiting further bleeding.
Methods:
Data Extraction
All patients who presented with aSAH between 2016 and 2020 at a single institution were retrospectively identified. Chart review was performed via electronic patient records, and data was extracted radiology reports, medication lists, progress notes, and discharge summaries with approval of our institutional review board. As this study was retrospective in nature, informed consent was not required.
Patient demographics were collected, including age, gender, race, and body mass index (BMI). Clinical presentation was assessed via Hunt Hess score and Glasgow Coma Scale. Pertinent comorbidities noted included intraventricular hemorrhage (IVH), intracerebral hemorrhage (ICH), hydrocephalus, and emergent pre-operative placement of an external ventricular drain (EVD). Use of anti-coagulant or anti-platelet medications during the pre-operative period was also noted. Procedural characteristics noted include technique (surgical clipping or endovascular coiling) and number of aneurysms treated. Post-operatively, the initiation time and dosage of subcutaneous unfractionated heparin were recorded. Time to heparinization was calculated as the difference between admission time and time of heparin administration. Our institutional protocol is to utilize both mechanical and chemical VTE prophylaxis post-operatively. All patients were confirmed to be wearing sequential compression devices for mechanical VTE prophylaxis for the duration of their admissions. Patients who presented with symptoms indicating possible DVT (e.g., leg swelling, leg pain) or PE (e.g., shortness of breath) were evaluated via imaging for definitive diagnosis. The presence of DVT was confirmed via Duplex ultrasonography and PEs were confirmed by CT scan. When possible, patient outcomes were assessed at one- and three-month follow-up using the Modified Rankin Scale (mRS). Analyses were restricted to individuals aged 18 years or older.
Statistical Analyses
aSAH patients with and without symptomatic venous thromboembolism (sVTE) were compared. Differences in categorical variables were computed using Chi-squared analysis, while those in continuous variables were assessed using non-parametric t tests. Significant variables were then assessed as a univariate predictor using logistic regression. Significant predictors from univariate analysis were incorporated into a multivariate logistic regression to identify significant factors. All analyses were performed using R version 4.2.0. A significance threshold of p<0.05 was applied in all analyses.
Results:
Demographic and Clinical Characteristics
194 patients were treated for aSAHs between 2016 and 2020, 33 of whom developed post-operative sVTE (25 DVTs and 14 PEs). Patients with and without sVTE were not statistically different in age, race, BMI, surgical technique (aneurysm clipping or endovascular coiling), number of aneurysms treated, incidence of vasospasm, pre-operative anti-coagulant/anti-platelet medication use, or use of aspirin or platelet infusions (Table 1). Men were more likely to present with sVTE than women (p=0.04).
Table 1.
Demographics and clinical characteristics of all patients who received treatment for aneurysmal subarachnoid hemorrhage from 2016–2020.
| No Symptomatic Thromboembolic Complications (n=161) | Symptomatic Thromboembolic Complications (n=33) | p-value | |
|---|---|---|---|
| Age (mean years) (SD) | 56.0 (14.2) | 55.5 (13.3) | 0.84 |
| Gender | 0.04 | ||
| Female (%) | 119 (74) | 18 (55) | |
| Male (%) | 42 (26) | 15 (45) | |
| Race | 0.53 | ||
| White (%) | 70 (43) | 15 (45) | |
| Black (%) | 59 (37) | 15 (45) | |
| Hispanic (%) | 15 (10) | 2 (6) | |
| Other (%) | 15 (10) | 1 (3) | |
| BMI (mean) (SD) | 28.2 (6.9) | 26.5 (8.9) | 0.31 |
| Hunt Hess Score | <0.01 | ||
| 2 or less (%) | 87 (51) | 9 (27) | |
| >2 (%) | 74 (49) | 24 (73) | |
| Glasgow Coma Scale | <0.01 | ||
| >12 (%) | 114 (71) | 14 (42) | |
| 8 to 12 (%) | 17 (11) | 11 (33) | |
| <8 (%) | 30 (19) | 8 (24) | |
| IVH (%) | 103 (64) | 27 (82) | 0.07 |
| ICH (%) | 37 (23) | 14 (42) | 0.04 |
| Hydrocephalus requiring EVD (%) | 59 (37) | 25 (76) | <0.01 |
| Craniotomy (%) | 72 (45) | 18 (56) | 0.40 |
| Coiling (%) | 86 (53) | 15 (45) | 0.52 |
| Mechanical Ventilation (%) | 54 (34) | 22 (67) | <0.01 |
| Pre-operative anti-coagulant or anti-platelet use (%) | 27 (17) | 8 (24) | 0.44 |
| Received Platelet Infusion (%) | 34 (21) | 12 (36) | 0.10 |
| Number of aneurysms treated | 0.47 | ||
| 1 (%) | 130 (81) | 29 (88) | |
| >1 (%) | 31 (19) | 4 (12) | |
| Received ASA post-operatively (%) | 55 (34) | 7 (21) | 0.21 |
| New hemorrhage post-operatively (%) | 11 (7) | 6 (18) | 0.08 |
| Time to heparin administration from admission (median hours) (SD) | 52.0 (31.0) | 59.9 (24.4) | 0.80 |
| Heparin Dose Administered | 0.17 | ||
| 5000 U SQ every 12 hours (%) | 42 (26) | 8 (24) | |
| 5000 U SQ every 8 hours (%) | 115 (71) | 22 (67) | |
| Other (%) | 4 (2) | 3 (9) | |
| Delayed heparinization (%) | 72 (46) | 23 (70) | 0.02 |
BMI, body mass index; EVD, external ventricular drain; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; SD, standard deviation; SQ, subcutaneously
Bolded values indicate statistical significance (p<0.05)
More severe aSAHs as measured by Hunt Hess score and Glasgow Coma scale were significantly associated with sVTE (both p<0.01). Additionally, patients initially presenting with ICH (p=0.04) but not IVH (p=0.07) were more likely to have a sVTE. Incidence of additional hemorrhages post-operatively was not significantly linked to sVTE. sVTE was more likely among patients who were placed on ventilation (p<0.01) and those presenting with hydrocephalus requiring EVD (p<0.01).
The number of hours from initial hospital admission to first usage of subcutaneous heparin were calculated for each patient, with a median time of 53.7 hours. Patients were dichotomized into the lower 50% (early heparin) and upper 50% (delayed heparin) of times to heparinization. Patients in the delayed heparin group were significantly more likely to present with sVTE than in those in the delayed group (p=0.02) (Figure 1a). There was no significant difference in heparin regiment (e.g., twice per day, thrice per day) between patients with and without sVTE.
Figure 1.
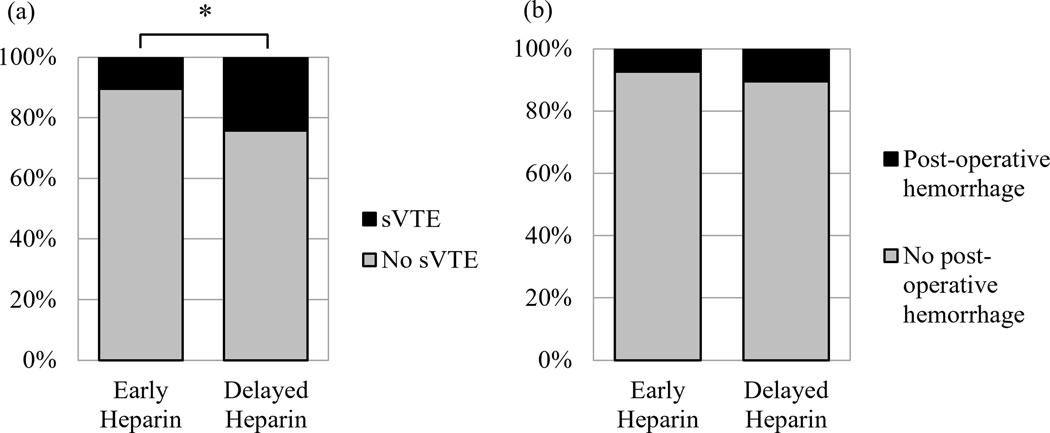
Patients who sustained delayed heparin initiation had a significantly higher rate of symptomatic VTE than those who began heparin earlier during their admission (a), but they exhibited no difference in the rate of post-operative hemorrhage (b). The asterisk denotes a statistically significant difference (p<0.05).
Univariate Predictors for Thromboembolic Complications
Metrics shown to be significant or near-significant via chi-squared analyses were further characterized using univariable logistic regression (Table 2). Male patients were significantly more likely to develop sVTE (odds ratio [OR] 2.36, 95% confidence interval [CI] 1.08–5.11, p=0.03). Patients with more severe clinical presentation at admission, as measured by the Hunt Hess (OR 1.46, CI 1.10–1.96, p=0.01) and Glasgow Coma (OR 0.90, CI 0.82–0.98, p=0.02) scales were also more likely to present with a sVTE. GCS was omitted from univariate analysis due to redundancy with the Hunt Hess scale. ICH (OR 2.47, CI 1.12–5.39, p=0.02) but not IVH (OR 2.53, CI 0.99–6.50, p=0.05) was associated with sVTE. Both hydrocephalus requiring EVD (OR 5.40, CI 2.38–13.51, p<0.01) and mechanical ventilation use (OR 3.96, CI 1.83–9.06, p<0.01) were also risk factors for sVTE. Delayed heparinization was also associated with higher incidence of sVTE (OR 2.72, CI 1.24–6.32, p=0.02).
Table 2.
Univariate logistic regression analysis of all risk factors associated with symptomatic venous thromboembolism in patients treated for aneurysmal subarachnoid hemorrhage.
| OR (95% CI) | p-value | |
|---|---|---|
| Gender (male) | 2.36 (1.08, 5.11) | 0.03 |
| Hunt Hess Grade | 1.46 (1.10, 1.96) | 0.01 |
| Glasgow Coma Scale | 0.90 (0.82, 0.98) | 0.02 |
| IVH | 2.53 (0.99, 6.50) | 0.05 |
| ICH | 2.47 (1.12, 5.39) | 0.02 |
| Hydrocephalus requiring EVD | 5.40 (2.38, 13.51) | <0.01 |
| Requiring mechanical ventilation | 3.96 (1.83, 9.06) | <0.01 |
| Delayed heparinization | 2.72 (1.24, 6.32) | 0.02 |
CI, confidence interval; EVD, external ventricular drain; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; OR, odds ratio
Bolded values indicate statistical significance (p<0.05)
Multivariate Logistic Regression for Thromboembolic Complications
Significant characteristics from univariate analysis were included in a multivariate logistic regression model to determine which factors independently predicted sVTE (Table 3). Hydrocephalus requiring EVD (OR 4.15, CI 1.57–11.90, p=0.01) and mechanical ventilation use (OR 2.91, CI 1.18–7.44, p=0.02) were independently associated with sVTE. Delayed heparinization showed a trend-level independent association with increased incidence of sVTE (OR 2.29, CI 0.95–5.90, p=0.07).
Table 3.
Multivariate logistic regression analysis of all risk factors associated with symptomatic venous thromboembolism in patients treated for aneurysmal subarachnoid hemorrhage.
| OR (95% CI) | p-value | |
|---|---|---|
| Gender (male) | 2.01 (0.84, 4.74) | 0.11 |
| Hunt Hess Grade | 0.91 (0.51, 1.62) | 0.76 |
| Glasgow Coma Scale | 1.01 (0.85, 1.20) | 0.87 |
| ICH | 1.52 (0.60, 3.77) | 0.37 |
| Hydrocephalus requiring EVD | 4.15 (1.57, 11.90) | 0.01 |
| Requiring mechanical ventilation | 2.91 (1.18, 7.44) | 0.02 |
| Delayed heparinization | 2.29 (0.95, 5.90) | 0.07 |
CI, confidence interval; EVD, external ventricular drain; ICH, intracerebral hemorrhage; OR, odds ratio
Bolded values indicate statistical significance (p<0.05)
Venous Thromboembolism, Heparin, and Patient Outcomes
sVTE was associated with worse patient prognosis. Those who developed sVTE displayed poorer outcomes as measured by mRS scores at both one- (p<0.01) and three- (p=0.02) month follow-up (Table 4). Patients with sVTE also had significantly longer hospital stays, defined as time from admission to discharge, than those without (p<0.01). Early heparin was not significantly associated with a higher incidence of post-operative hemorrhage (Figure 1b).
Table 4.
Effect of symptomatic venous thromboembolism on length of hospital stay and outcomes as measured by mRS at one- and three-month follow-up visit.
| No Symptomatic Thromboembolic Complications | Symptomatic Thromboembolic Complications | p-value | |
|---|---|---|---|
| mRS, 1 month follow-up | <0.01 | ||
| 2 or less (%) | 93 (58) | 7 (22) | |
| >2 (%) | 68 (42) | 26 (78) | |
| mRS, 3 months follow-up | 0.02 | ||
| 2 or less (%) | 87 (66) | 12 (40) | |
| >2 (%) | 45 (34) | 18 (60) | |
| Length of hospital stay | <0.01 | ||
| 21 days or less (%) | 101 (63) | 6 (18) | |
| >21 days (%) | 60 (37) | 27 (82) |
mRS, Modified Rankin scale
Bolded values indicate statistical significance (p<0.05)
Discussion:
Those patients who survive initial treatment for aSAH face a prolonged recovery with great risk of mortality and further disability. VTE increases the risk of death among neurosurgical patients, with estimates of the mortality rate of PE ranging from 9 to 50%.5 Our work shows that patients with symptomatic thromboembolic complications were significantly more likely to present with worse outcomes several months following aneurysm rupture. We also found that sVTE was associated with longer hospital stays, a well-studied relationship that poses significantly higher costs to patients.6, 10, 11 Thromboembolic complications clearly pose harm to aSAH patients, and there is need for prophylactic strategies.
A robust understanding of risk factors is important in predicting those patients most likely to develop thromboembolic complications. Like previous work, we show that male sex is an independent predictor of sVTE among aSAH patients.6 In the general population, incidence of DVT is elevated in men, and our work suggests that this discrepancy holds among those treated for aSAH.12 Clinical SAH severity and incidence of intracerebral hemorrhage were found to be significantly associated with the development of sVTE on univariate analysis. Our results match those from similar studies which find that aSAH patients with more severe disease presentations have increased incidence of sVTE.13 Interestingly, most factors related to neurosurgical clinical course (procedural technique, vasospasm, intraoperative platelet use) were not significant predictors of sVTE. A notable exception includes EVD placement, which has previously been linked to sVTE and may be a proxy for clinical severity.4, 14, 15 The use of mechanical ventilation by patients in our cohort was also linked to increased risk of sVTE, likely due to decreased mobilization during recovery. Increased mobilization has been found to decrease incidence of vasospasm and sVTE, including among patients with aSAH.4, 14, 16–18
Chemical prophylaxis is another important strategy in mitigating the risk of VTE. Prophylactic heparin has been shown to reduce the risk of DVT without increasing the risk of bleeding events or death in aSAH.4, 19–21 Yet there is limited research as to the appropriate timing of heparin introduction in this population. Though it is clear that the efficacy of prophylactic heparin is time-dependent, there is some concern that early administration may increase the risk of intracranial hemorrhage.17, 22 Certainly some concern is warranted, as initiation of heparin within four hours of ventriculostomy placement significantly increased the odds of tract hemorrhage in a cohort of patients with aSAH requiring external ventricular drain placement.9 Moreover, prophylactic unfractionated heparin use in neurotrauma patients has been shown effective in decreasing thromboembolisms while not increasing the rate of hemorrhagic complications.23 Among other neurosurgical trauma populations, delayed initiation of chemoprophylaxis has been shown to increase the risk of VTE.15, 24
However, there is little to no direct evidence elucidating this relationship between heparin timing and VTE rates among aSAH patients. We find that patients with aSAH show increased risk of sVTE when heparin is delayed after admission. Other work in aSAH also found that heparin use within 24 hours of the treatment of ruptured aneurysm was generally safe, though this group did not characterize development of VTE.25 Overall, early initialization appears to be important in maintaining the efficacy of prophylactic heparin among patients with aSAH, while the risk of hemorrhagic complication is low. Nevertheless, a thorough review of patient history for risk factors, including thrombocytopenia, antiplatelet agents, and renal replacement therapy, is also warranted to mitigate complications of heparin thromboprophylaxis.26
This study has several limitations. First, the retrospective nature of our study does not permit the randomization of patients with aSAH to different heparin initiation times. It also prevents us from determining causality with our risk factors. This study also encompasses the experience of patients at a single institution over five years. A larger, multi-institutional cohort of patients may be required to validate these findings. Additionally, there were only 33 patients presenting with sVTE in our study population. Our multivariate logistic model utilizes seven variables, with an event-per-variable ratio of 4.7. Prior work suggests that studies with lower event-per-variable ratios may be subject to more bias in calculating regression coefficients.27 Thus, future work with larger study populations may be required to validate this multivariate risk model. Lastly, at our institution, the decision of when to initiate and how to dose heparin rests on the surgical and medical teams. These decisions incorporate physician experience alongside patient presentation, which may incorporate bias into when heparin was administered. Regardless, the present study may help guide providers in determining the risk of sVTE among patients with aSAH and guide them on the appropriate use of prophylactic anti-coagulation.
Conclusion:
Prophylactic heparin therapy is effective in reducing the risk of VTE – a common finding among patients with aSAH. We show that in 194 cases, our sVTE rate was 17%. Patients with sVTE had longer hospital stays and worse outcomes at both one- and three-month follow-up visits. Significant sVTE risk factors on univariate analysis included male sex, Hunt Hess score, Glasgow Coma scale, ICH, hydrocephalus requiring EVD, and use of mechanical ventilation. Only hydrocephalus requiring EVD and use of mechanical ventilation remained significant upon multivariate analysis, while delayed heparinization showed a trend-level independent association with sVTE We also find that aSAH who experience a delay in the initiation of heparin prophylaxis are significantly more likely to develop sVTE. Through careful evaluation of patient risk factors and deliberately timed heparin administration, VTE may be reduced among patients with aSAH.
Financial Support:
Author C.B.K receives tuition and stipend support from the Medical Scientist Training Program at the Johns Hopkins University School of Medicine (NIH/NIGMS T32GM007309).
Abbreviations:
- aSAH
aneurysmal subarachnoid hemorrhage
- BMI
Body Mass Index
- CI
confidence interval
- DVT
deep vein thrombosis
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- mRS
Modified Rankin Scale
- OR
odds ratio
- PE
pulmonary embolism
- sVTE
symptomatic venous thromboembolism
- VTE
venous thromboembolism
Footnotes
Disclosures: The authors do not declare any conflicts of interest.
REFERENCES
- 1.Neifert SN, Chapman EK, Martini ML, et al. Aneurysmal Subarachnoid Hemorrhage: the Last Decade. Translational Stroke Research. 2021;12(3):428–446. doi: 10.1007/s12975-020-00867-0. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN. Management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2009;37(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray WZ, Strom RG, Blackburn SL, Ashley WW, Sicard GA, Rich KM. Incidence of deep venous thrombosis after subarachnoid hemorrhage. J Neurosurg. 2009;110(5):1010–1014. doi: 10.3171/2008.9.JNS08107 [doi]. [DOI] [PubMed] [Google Scholar]
- 4.Liang CW, Su K, Liu JJ, Dogan A, Hinson HE. Timing of deep vein thrombosis formation after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2015;123(4):891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton MG, Hull RD, Pineo GF. Venous thromboembolism in neurosurgery and neurology patients: a review. Neurosurgery. 1994;34(2):280–296. [DOI] [PubMed] [Google Scholar]
- 6.Kshettry VR, Rosenbaum BP, Seicean A, Kelly ML, Schiltz NK, Weil RJ. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. Journal of clinical neuroscience. 2014;21(2):282–286. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MG, Yee WH, Hull RD, Ghali WA. Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery. 2011;68(3):571–581. [DOI] [PubMed] [Google Scholar]
- 8.Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of Venous Thromboembolism in Neurosurgery: A Metaanalysis. Chest. 2008;134(2):237–249. doi: 10.1378/chest.08-0023. [DOI] [PubMed] [Google Scholar]
- 9.Gard AP, Sayles BD, Robbins JW, Thorell WE, Surdell DL. Hemorrhage rate after external ventricular drain placement in subarachnoid hemorrhage: time to heparin administration. Neurocritical Care. 2017;27(3):350–355. [DOI] [PubMed] [Google Scholar]
- 10.Mouchtouris N, Lang MJ, Barkley K, et al. Predictors of hospital-associated complications prolonging ICU stay in patients with low-grade aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;132(6):1829–1835. [DOI] [PubMed] [Google Scholar]
- 11.Alaraj A, Hussein AE, Esfahani DR, Amin-Hanjani S, Aletich VA, Charbel FT. Reducing length of stay in aneurysmal subarachnoid hemorrhage: a three year institutional experience. Journal of Clinical Neuroscience. 2017;42:66–70. [DOI] [PubMed] [Google Scholar]
- 12.Bauersachs RM, Riess H, Hach-Wunderle V, et al. Impact of gender on the clinical presentation and diagnosis of deep-vein thrombosis. Thromb Haemost. 2010;103(04):710–717. [DOI] [PubMed] [Google Scholar]
- 13.Unda SR, Labagnara K, Birnbaum J, et al. Impact of hospital-acquired complications in long-term clinical outcomes after subarachnoid hemorrhage. Clin Neurol Neurosurg. 2020;194:105945. [DOI] [PubMed] [Google Scholar]
- 14.Rennert RC, Martin JR, Brandel MG, et al. Risk factors for venous thromboembolism after admission for traumatic subdural hematoma at level I trauma center: large single-institution series. World Neurosurgery. 2019;122:e619–e626. [DOI] [PubMed] [Google Scholar]
- 15.Geraldini F, De Cassai A, Correale C, et al. Predictors of deep-vein thrombosis in subarachnoid hemorrhage: a retrospective analysis. Acta Neurochir. 2020;162(9):2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karic T, Røe C, Nordenmark TH, Becker F, Sorteberg W, Sorteberg A. Effect of early mobilization and rehabilitation on complications in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126(2):518–526. [DOI] [PubMed] [Google Scholar]
- 17.Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151(3):180–190. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CM, Overend TJ, Godwin J, Sealy C, Sunderji A. Ambulation after deep vein thrombosis: a systematic review. Physiotherapy Canada. 2009;61(3):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browd SR, Ragel BT, Davis GE, Scott AM, Skalabrin EJ, Couldwell WT. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurgical focus. 2004;17(4):1–6. [DOI] [PubMed] [Google Scholar]
- 20.Hacker RI, Ritter G, Nelson C, et al. Subcutaneous heparin does not increase postoperative complications in neurosurgical patients: an institutional experience. J Crit Care. 2012;27(3):250–254. [DOI] [PubMed] [Google Scholar]
- 21.Epstein NE. A review of the risks and benefits of differing prophylaxis regimens for the treatment of deep venous thrombosis and pulmonary embolism in neurosurgery. Surg Neurol. 2005;64(4):295–301. [DOI] [PubMed] [Google Scholar]
- 22.Gnanalingham KK, Holland JP. Attitudes to the use of prophylaxis for thrombo-embolism in neurosurgical patients. Journal of clinical neuroscience. 2003;10(4):467–469. [DOI] [PubMed] [Google Scholar]
- 23.Iyama K, Ikeda S, Inokuma T, et al. How to Safely Prevent Venous Thromboembolism in Severe Trauma Patients A Novel Protocol to Prevent Trauma-Related Venous Thromboembolism. International Heart Journal. 2020;61(5):993–998. [DOI] [PubMed] [Google Scholar]
- 24.Tracy BM, Dunne JR, O’Neal CM, Clayton E. Venous thromboembolism prophylaxis in neurosurgical trauma patients. J Surg Res. 2016;205(1):221–227. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira Manoel AL, Turkel-Parrella D, Germans M, et al. Safety of early pharmacological thromboprophylaxis after subarachnoid hemorrhage. Canadian Journal of Neurological Sciences. 2014;41(5):554–561. [DOI] [PubMed] [Google Scholar]
- 26.Lauzier F, Arnold DM, Rabbat C, et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med. 2013;39(12):2135–2143. [DOI] [PubMed] [Google Scholar]
- 27.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. [DOI] [PubMed] [Google Scholar]


