Abstract
Introduction
Targeted low-dose CT lung cancer screening reduces lung cancer mortality. England’s Targeted Lung Health Check programme uses risk prediction tools to determine eligibility for biennial screening among people with a smoking history aged 55–74. Some participants initially ineligible for lung cancer screening will later become eligible with increasing age and ongoing tobacco exposure. It is, therefore, important to understand how many people could qualify for reinvitation, and after how long, to inform implementation of services.
Methods
We prospectively predicted future risk (using Prostate, Lung, Colorectal and Ovarian trial’s risk model (PLCOm2012) and Liverpool Lung Project version 2 (LLPv2) risk models) and time-to-eligibility of 5345 participants to estimate how many would become eligible through the course of a Lung Health Check screening programme for 55–74 years.
Results
Approximately a quarter eventually become eligible, with those with the lowest baseline risks unlikely to ever become eligible. Time-to-eligibility is shorter for participants with higher baseline risk, increasing age and ongoing smoking status. At a PLCOm2012 threshold ≥1.51%, 68% of those who continue to smoke become eligible compared with 18% of those who have quit.
Discussion
Predicting which participants may become eligible, and when, during a screening programme can help inform reinvitation strategies and service planning. Those with risk scores closer to the eligibility threshold, particularly people who continue to smoke, will reach eligibility in subsequent rounds while those at the lowest risk may be discharged from the programme from the outset.
Keywords: Lung Cancer, Mass Screening, Clinical Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
There has been a lack of data describing individuals who are deemed, at baseline, at too low a risk of lung cancer to be eligible for targeted screening.
WHAT THIS STUDY ADDS
From a large real-world cohort, we show that time-to-eligibility can be predicted at baseline risk assessment, dependent on smoking status.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
With lung cancer screening being implemented internationally, these findings can inform reinvitation strategies to support equitable service delivery. We identify potential risk thresholds that could be applied to guide the timing of reinvitation.
Introduction
Targeted lung cancer screening is being implemented in the UK following a recommendation by the UK National Screening Committee.1 The Targeted Lung Health Check (TLHC) programme in England currently uses two lung cancer risk prediction models to determine eligibility for low-dose CT (LDCT) screening among people aged 55–74 years with a smoking history: the Prostate, Lung, Colorectal and Ovarian trial’s risk model (PLCOm2012), set at a 6-year lung cancer risk of ≥1.51% and the Liverpool Lung Project version 2 (LLPv2) model, set at a 5-year risk of ≥2.5%.2–4 This approach differs from other countries, such as the USA, where age and smoking history alone (age 50–80, ≥20 pack-year smoking history and quitting smoking within 15 years) are used as categorical eligibility criteria.5 Risk rises with age and smoking exposure, so some invitees who fall below these risk thresholds at the point of initial assessment will become eligible before reaching age 75. It is, therefore, important to understand how many people could qualify for reinvitation, and after how long, to inform participants of their likelihood of qualifying for lung cancer screening in the future, and to plan for longer-term national implementation of lung cancer screening.
Methods
Lung cancer risk assessment data from two Greater Manchester Lung Health Check (LHC) programmes were collected prospectively in a bespoke clinical database. Details of the programmes, which commenced in 2016 and 2019, have been described previously.6 7 In brief, people with a smoking history aged 55–74 and registered at participating primary care practices were invited to attend free LHCs in community-based mobile units. As part of the LHC, future lung cancer risk was calculated using the PLCOm2012 risk model, with individuals scoring ≥1.51% being offered immediate colocated LDCT screening.
For this study, lung cancer risk scores were forecasted for 20 subsequent years, accounting for increasing age while assuming other risk model variables (eg, smoking status, diagnosis of lung disease) do not change. For participants who were initially ineligible for screening, we predicted the year at which the eligibility threshold may be crossed. Modelling was performed to show how many initially ineligible participants become eligible at each year over the proposed screening age range of 55–74 years. Participants were ineligible after reaching age 75y. The number of participants becoming eligible, and after how many years, was calculated with stratification by baseline risk and by age. Analysis was performed for eligibility by PLCOm2012 alone, LLPv2 alone, and then for eligibility by either PLCOm2012 or LLPv2, as per the TLHC’s current eligibility criteria.2 Data were then stratified by smoking status at the time of baseline assessment, as risk scores increase at different rates over time depending on ongoing smoking exposure.
Next, we estimated the number of lung cancer cases predicted to arise prior to the point of reaching eligibility by each risk threshold. This was done by multiplying the baseline predicted lung cancer risk for each participant by their time-to-eligibility in years, divided by the prediction period used by each risk model (6 years for PLCOm2012, 5 years for LLPv2). The sum of these risks represents the predicted number of lung cancers that may arise prior to eligibility for screening.
Potential pragmatic reinvitation time points for a biennial lung cancer screening programme were explored, with a description of how many participants in various baseline risk strata may have reached eligibility by each time point. Time points were derived from the inspection of time-to-eligibility groupings using PLCOm2012.
Lung cancer risk trajectories for participants with selected baseline PLCOm2012 and LLPv2 scores across the ineligible range were then displayed. For participants with each example baseline risk score, mean risks were calculated for subsequent years to display the point at which screening eligibility may be reached, according to smoking status. Analyses were performed in R V.4.2.
Patient and public involvement
Patient and public involvement was sought through a focus group, facilitated by the Manchester National Institute for Health and Care Research Biomedical Research Centre (NIHR BRC), to discuss the use of data from LHCs. There was positive support for performing analyses to discover how screening delivery may be optimised and to share these findings with the scientific community.
Results
PLCOm2012 approach
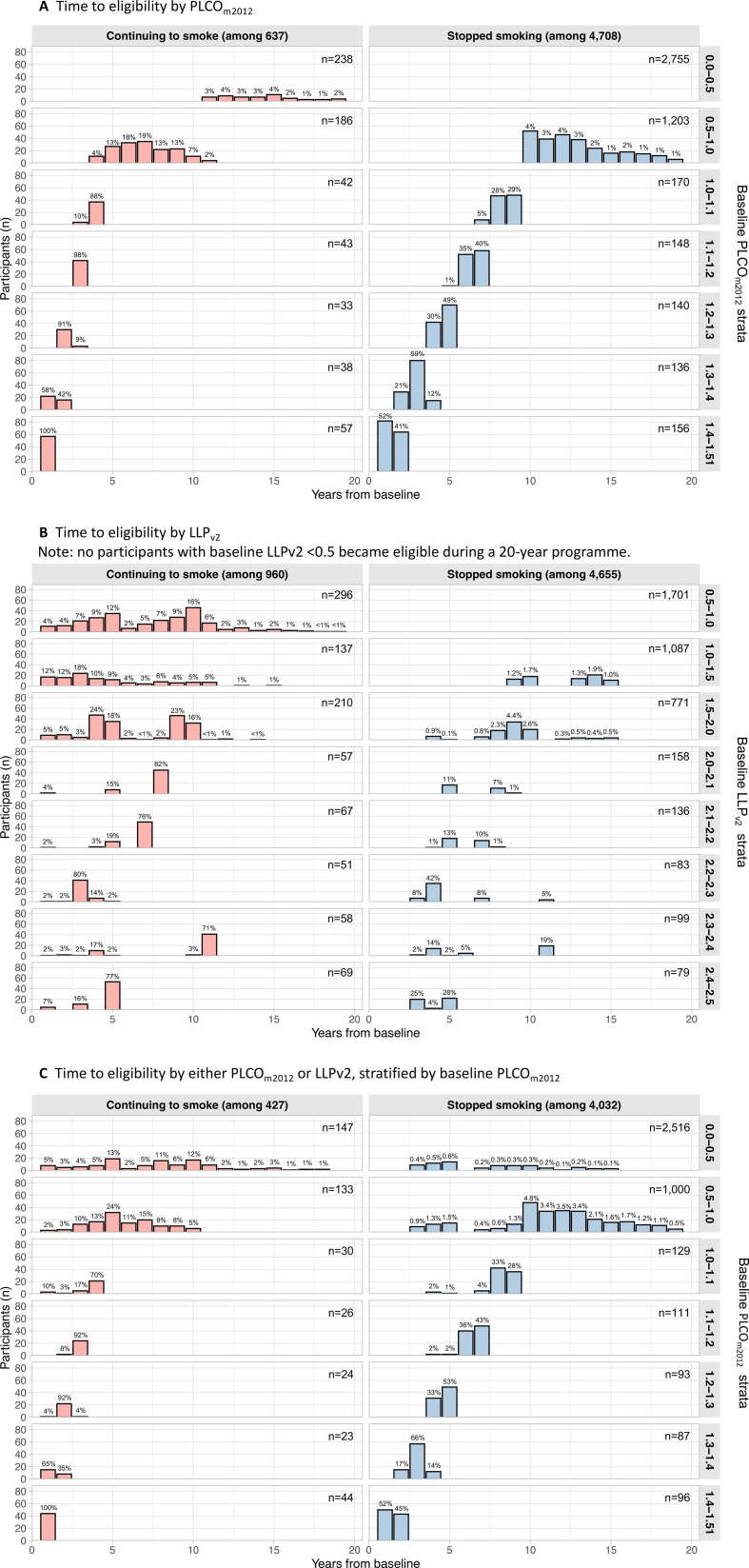
In a cohort of 10 299 LHC attendees, 52% (n=5345) were ineligible at baseline with PLCOm2012<1.51%, of which almost 1 in 4 (24%; n=1295/5345) were predicted to become eligible during a screening programme for 55–74 year-olds. A breakdown by baseline risk is shown in table 1. Only 1.9% of those at lowest risk (PLCOm2012<0.5%) ever became eligible, all of whom were aged ≤62 years at baseline, whereas 84% of those at PLCOm2012 1.0%–1.51% became eligible. 95% of those just below eligibility (1.4%–1.51%) became eligible after a median of 1 (IQR 1–2) year. Based on baseline PLCOm2012 scores, lung cancer was predicted to arise in 1.0% of those becoming eligible before age 75 (n=13/1295) prior to reaching the eligibility threshold. The timing of eligibility stratified by smoking status is shown in figure 1. 68% of current smokers (n=433/637) were predicted to become eligible if they continued to smoke the same amount while 18% of former smokers would ever become eligible if they remained abstinent from tobacco. In the lowest PLCOm2012 risk group (<0.5%), former smokers never became eligible, and active smokers took >10 years to become eligible. In those scoring 0.5%–1.0%, the median time to eligibility in former smokers was 12 (11–15) years, with none becoming eligible prior to 10 years, and 7 (6–8) years in current smokers.
Table 1.
Participants becoming eligible by PLCOm2012 alone or by either PLCOm2012 or LLPv2, stratified by baseline PLCOm2012 score
| Baseline PLCOm2012 risk strata | |||||||
| 0.0–0.5 | 0.5–1.0 | 1.0–1.1 | 1.1–1.2 | 1.2–1.3 | 1.3–1.4 | 1.4–1.51 | |
| LHC attendees, n | 2993 | 1389 | 212 | 191 | 173 | 174 | 213 |
| LHC attendees becoming eligible by PLCOm2012 | 56 (1.9%) | 432 (31%) | 144 (68%) | 153 (80%) | 145 (84%) | 162 (93%) | 203 (95%) |
| Years to eligibility by PLCOm2012, median (IQR) | 14 (12–16) | 11 (8–13) | 8 (4–9) | 6 (3–7) | 4 (4–5) | 3 (2–3) | 1 (1–2) |
| Age at becoming eligible by PLCOm2012, median (IQR) | 71 (70–73) | 69 (66–72) | 68 (64–71) | 66 (63–70) | 67 (62–70) | 65 (61–69) | 63 (59–69) |
| Cancers predicted to arise before reaching eligibility by PLCOm2012 | 0.4 (0.8%) | 6.2 (1.4%) | 1.8 (1.2%) | 1.6 (1.1%) | 1.2 (0.8%) | 0.9 (0.6%) | 0.6 (0.3%) |
| LHC attendees ineligible at baseline by either PLCOm2012 or LLPv2, n | 2663 | 1133 | 159 | 137 | 117 | 110 | 140 |
| LHC attendees becoming eligible by PLCOm2012 or LLPv2 | 204 (7.7%) | 423 (37%) | 117 (74%) | 118 (86%) | 104 (89%) | 107 (97%) | 137 (98%) |
| Years to eligibility by PLCOm2012 or LLPv2, median (IQR) | 8 (5–10) | 10 (6–13) | 8 (4–9) | 6 (5–7) | 4 (4–5) | 3 (2–3) | 1 (1–2) |
| Age at becoming eligible by PLCOm2012 or LLPv2, median (IQR) | 65 (65–70) | 68 (65–71) | 67 (63–70) | 65 (62–69) | 66 (61–70) | 63 (60–69) | 61 (58–67) |
| Cancers predicted to arise before reaching eligibility by PLCOm2012 or LLPv2 | 0.5 (0.3%) | 5.4 (1.3%) | 1.4 (1.2%) | 1.3 (1.1%) | 0.9 (0.8%) | 0.6 (0.6%) | 0.4 (0.3%) |
LHC, Lung Health Check; LLPv2, Liverpool Lung Project version 2; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
Figure 1.
LHC attendees who were ineligible at baseline by differing criteria (A–C), stratified by baseline risk score and smoking status. Percentages describe the proportion of participants in each risk stratum in each smoking status group who become eligible at each time point. (A) Becoming eligible by PLCOm2012. (B) Becoming eligible by LLPv2. No participants with baseline LLPv2<0.5 became eligible during a 20-year programme. (C) Becoming eligible by either PLCOm2012 or LLPv2, stratified by baseline PLCOm2012. LHC, Lung Health Check; LLPv2, Liverpool Lung Project version 2; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
LLPv2 approach
Using LLPv2 at threshold ≥2.5%, 55% of participants (n=5615/10 299) would have been ineligible at baseline, of whom 23% (n=1265/5615) were predicted to ever become eligible during a screening programme of 55–74 years screening programme, with detail shown in table 2. Based on baseline LLPv2 scores, lung cancer was predicted to occur in 2.2% of those becoming eligible before age 75 (n=28/1265) prior to reaching the eligibility threshold; more than those predicted by PLCOm2012 as above (two-tailed z score p=0.015). Nobody with baseline LLPv2<0.5% was predicted to ever become eligible, nor were any people who quit smoking with LLPv2<1.0%. As shown in figure 1B, there was a wider distribution of newly eligible cases arising in each risk stratum.
Table 2.
Participants becoming eligible by LLPv2 alone or by either PLCOm2012 or LLPv2, stratified by baseline LLPv2 score
| Baseline LLPv2 risk strata | |||||||||
| 0.0–0.5 | 0.5–1.0 | 1.0–1.5 | 1.5–2.0 | 2.0–2.1 | 2.1–2.2 | 2.2–2.3 | 2.3–2.4 | 2.4–2.5 | |
| LHC attendees, n | 556 | 1997 | 1224 | 981 | 215 | 203 | 134 | 157 | 148 |
| LHC attendees becoming eligible by LLPv2 | 0 (0%) | 269 (13%) | 200 (16%) | 295 (30%) | 85 (40%) | 99 (49%) | 104 (78%) | 99 (63%) | 114 (77%) |
| Years to eligibility by LLPv2, median (IQR) | – | 8 (4–10) | 8 (3–11) | 9 (4–9) | 8 (5–8) | 7 (5–7) | 4 (3–4) | 11 (4–11) | 5 (3–5) |
| Age at becoming eligible by LLPv2, median (IQR) | – | 65 (60–65) | 65 (62–70) | 65 (60–67) | 65 (65–65) | 65 (65–65) | 60 (60–65) | 70 (65–70) | 65 (65–65) |
| Cancers predicted to arise before reaching eligibility by LLPv2 | – | 2.9 (1.1%) | 3.9 (1.9%) | 7.8 (2.6%) | 2.5 (2.9%) | 2.7 (2.7%) | 1.8 (1.8%) | 3.9 (3.9%) | 2.4 (2.1%) |
| LHC attendees ineligible at baseline by either PLCOm2012 or LLPv2, n | 556 | 1826 | 988 | 646 | 126 | 107 | 72 | 67 | 71 |
| LHC attendees becoming eligible by PLCOm2012 or LLPv2 | 15 (2.7%) | 461 (25%) | 282 (29%) | 232 (36%) | 48 (38%) | 46 (43%) | 51 (71%) | 31 (46%) | 44 (62%) |
| Years to eligibility by PLCOm2012 or LLPv2, median (IQR) | 9 (6–15) | 8 (4–12) | 7 (3–10) | 5 (3–8) | 5 (2–7) | 5 (3–7) | 3 (2–4) | 4 (3–5) | 3 (3–5) |
| Age at becoming eligible by PLCOm2012 or LLPv2, median (IQR) | 69 (64–72) | 65 (62–70) | 68 (65–70) | 66 (61–70) | 66 (64–70) | 65 (63–70) | 64 (60–65) | 65 (62–70) | 65 (64–65) |
| Cancers predicted to arise before reaching eligibility by PLCOm2012 or LLPv2 | 0.1 (0.8%) | 5.7 (1.2%) | 4.8 (1.7%) | 4.5 (1.9%) | 0.9 (1.8%) | 0.9 (2.1%) | 0.8 (1.6%) | 0.7 (2.1%) | 0.8 (1.8%) |
LHC, Lung Health Check; LLPv2, Liverpool Lung Project version 2; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
Either model approach
Using eligibility by either score (PLCOm2012 or LLPv2), 43% of participants (n=4459/10 299) would have been ineligible at baseline. 27% (n=1210/4459) of these were predicted to become eligible (tables 1 and 2), with shorter time-to-eligibility for modest numbers of participants as shown in figure 1C. Based on baseline risk scores, lung cancers were predicted to occur in 0.9% of those becoming eligible before age 75 (n=11/1210) using PLCOm2012 risks, or 1.6% (n=19/1210) using LLPv2 risks.
Eligibility stratified by age is displayed in table 3. The likelihood of ever becoming eligible reduces with increasing age at the time of risk assessment, as the time window to reach the age limit shortens. Median time-to-eligibility is shorter with each prediction model approach, reducing from 7–8 years to 2–4 years depending on the approach.
Table 3.
Participants becoming eligible by PLCOm2012 alone, by LLPv2 alone and by reaching eligibility by either score, stratified by age at baseline
| Age at baseline | ||||
| 55–59 years | 60–64 years | 65–69 years | 70–74 years | |
| LHC attendees, N | 2835 | 2677 | 2597 | 2190 |
| PLCOm2012 | ||||
| LHC attendees ineligible at baseline, n | 1839 | 1401 | 1169 | 936 |
| LHC attendees becoming eligible | 684 (37%) | 374 (27%) | 188 (16%) | 49 (5.2%) |
| Years to eligibility, median (IQR) | 7 (3–12) | 6 (3–9) | 4 (2–5) | 2 (1–3) |
| LLPv2 | ||||
| LHC attendees ineligible at baseline, n | 2312 | 1444 | 1119 | 740 |
| LHC attendees becoming eligible | 930 (40%) | 281 (19%) | 53 (4.7%) | 1 (0.1%) |
| Years to eligibility, median (IQR) | 8 (4–10) | 5 (4–7) | 4 (3–5) | 4 (4–4) |
| Either PLCOm2012 or LLPv2 | ||||
| LHC attendees ineligible at baseline, n | 1750 | 1175 | 917 | 617 |
| LHC attendees becoming eligible | 722 (41%) | 336 (29%) | 129 (14%) | 23 (3.7%) |
| Years to eligibility, median (IQR) | 7 (3–11) | 5 (3–9) | 4 (2–5) | 2 (1–3) |
LHC, Lung Health Check; LLPv2, Liverpool Lung Project version 2; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
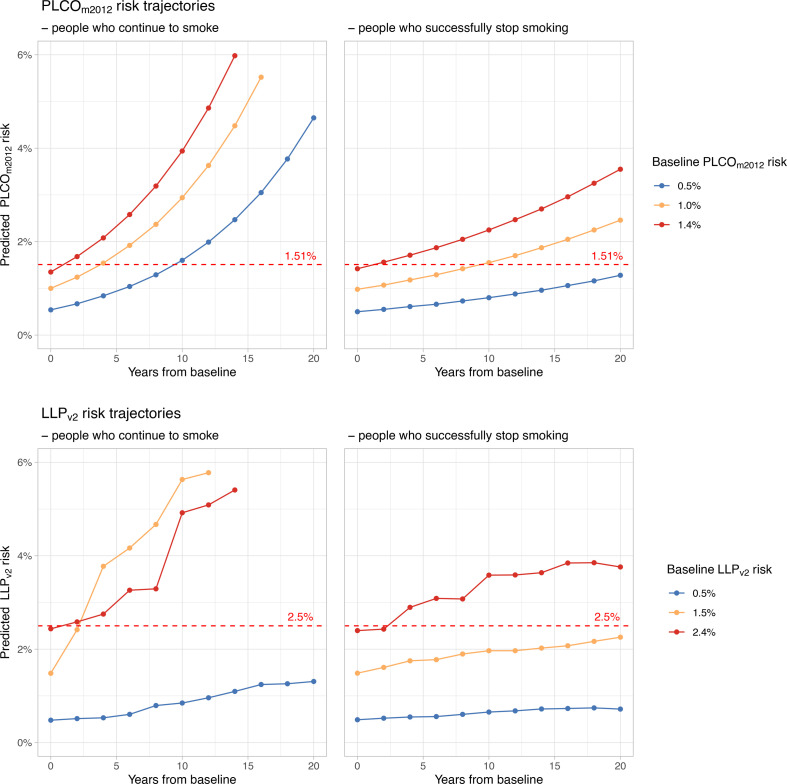
The visible groupings of time-to-eligibility by PLCOm2012 are reflected in table 4, where the majority of newly eligible participants were predicted to become eligible by certain rounds in a biennial programme. Projections of risk trajectories starting at a selection of illustrative baseline risks, stratified by smoking status, are presented in figure 2.
Table 4.
Example of when to reinvite participants to a biennial screening programme based on baseline PLCOm2012 scores and smoking status
| Baseline PLCOm2012 risk score category | When to consider reinviting people currently smoking | Proportion eligible by time of reinvitation (current), % (n/N) | When to consider reinviting people who formerly smoked | Proportion eligible by time of reinvitation (former), % (n/N) |
| <0.5% | 14 years | 54 (30/56) | Discharge | N/A (0/0) |
| 0.5%–1.0% | 8 years | 77 (128/166) | 12 years | 52 (137/266) |
| 1.0%–1.2% | 4 years | 100 (83/83) | 8 years | 78 (166/214) |
| 1.2%–1.4% | 2 years | 96 (68/71) | 4 years | 70 (166/236) |
| 1.4%–1.51% | 2 years | 100 (57/57) | 2 years | 100 (146/146) |
Presented with estimated proportions of participants in each risk category forecasted to become eligible by each proposed time of reinvitation; among N participants in each risk category who is forecasted to ever become eligible during a 20-year screening programme.
N/A, not available; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
Figure 2.
Lung cancer risk trajectories predicted by PLCOm2012 and LLPv2 according to smoking status. For participants with each example baseline risk score, mean risks were calculated over subsequent years to display when screening eligibility may be reached (crossing the dashed red line). The y-axes are limited to display the eligibility thresholds more clearly. LLPv2, Liverpool Lung Project version 2; PLCOm2012, Prostate, Lung, Colorectal and Ovarian trial’s risk model 2012.
Discussion
Our modelling predicts that approximately a quarter of initially ineligible LHC participants will become eligible for screening during a programme offered between the ages of 55 and 74 years, such as England’s TLHC programme. The PLCOm2012 model yielded an appreciable grouping of times-to-eligibility according to baseline risk. LLPv2 gave wider distributions of times-to-eligibility, attributable to the categorical nature of variables such as age and smoking history; as individuals have a range of ages at baseline, they pass into higher age categories in a stepwise manner, resulting in a wider range of time intervals for any given baseline risk. When screening eligibility is determined by crossing the risk threshold of either model, time-to-eligibility was brought forward and drew in participants from low-risk categories. The estimated cumulative risk of being diagnosed with lung cancer prior to reaching eligibility is below the TLHC’s risk threshold for each model (1.0% for PLCOm2012 and 2.2% for LLPv2) so the delay prior to reinvitation appears acceptable as screening aims to balance its benefits and harms.
The prediction of future eligibility, using real-world lung cancer risk prediction model scores, is novel and can potentially aid with national implementation. A limitation of this work is the assumption that risk factors remain constant. Tobacco consumption is particularly difficult to predict, and this is a major component of risk prediction models. Tobacco dependency interventions provided within screening programmes aim to help people quit tobacco, but some participants will change their smoking status in either direction, impacting the accuracy of forecasted risk. Our estimates of the number of cancers that may arise prior to reaching eligible risk thresholds are limited by the assumption that baseline risks of being diagnosed with lung cancer without screening remain constant over time periods different from those the risk models were developed to predict. These estimates also assume good model calibration. It has been recognised that the high risk Manchester cohorts first targeted for screening yielded more cancers than had been predicted.8
Equitable screening programmes should apply eligibility criteria consistently, including as time progresses and risk evolves. Risk prediction tools, such as PLCOm2012 and LLPv2, offer a means to identify time intervals at which to reinvite initially ineligible individuals for reassessment and potential enrolment into screening. Future work should focus on how best to implement this. Options include batch reinvitation of groups after specific time intervals based on baseline risk categories, as demonstrated in table 4, or individual reinvitation after predicting time to eligibility for each participant at their baseline assessment. Mechanisms could be explored to update participants’ risk predictions over time, such as through linkage to primary care records. For example, if smoking status changes or a new diagnosis of obstructive airways disease is made, predicted times-to-eligibility could be adjusted to further inform the timing of reinvitation. Notably, all participants with PLCOm2012≥1.4% at baseline are predicted to become eligible in 2 years whether they smoke or not, meaning that they could simply be invited for an LDCT at the next screening round rather than undergoing reassessment. Such forward planning could improve uptake, streamline programme planning, and simplify the message delivered to participants.
In conclusion, our results demonstrate that approximately a quarter of initially ineligible individuals will become eligible for lung cancer screening before the age of 75 and their time-to-eligibility can be estimated based on baseline risk.
Footnotes
@patrick_goodley, @ProfPhilCrosbie, @MatthewSperrin, @ZoeMerchantOT, @booton_r, @hsbalata
Contributors: This study was conceptualised by HB and PG. Data were handled, analysed and presented by PG, with input from HB and MS. The manuscript was written by PG and HB. All authors contributed to and approved the final manuscript. HB is the guarantor.
Funding: PG receives a PhD studentship from the North West Lung Centre Charity. PAJC is supported by the National Institute for Health Research Manchester Biomedical Research Centre (IS-BRC-1215-20007).
Competing interests: RB has received honoraria for educational events by Siemens Healthineers and Cobalt Medical Imaging.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Data and analysis code are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was an evaluation of a clinical service. This evaluation was approved by the Manchester LHC Steering Committee. Full research ethics committee approval was not required.
References
- 1. UK National Screening Committee . Adult screening programme: lung cancer. 2022. Available: https://view-health-screening-recommendations.service.gov.uk/lung-cancer/ [Accessed May 2022].
- 2. NHS England, National Cancer Programme . Targeted screening for lung cancer with low radiation dose computed tomography: standard protocol prepared for the targeted lung health checks programme. 2 Ed. 2022. [Google Scholar]
- 3. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728–36. 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Field JK, Vulkan D, Davies MPA, et al. Liverpool lung project lung cancer risk stratification model: calibration and prospective validation. Thorax 2021;76:161–8. 10.1136/thoraxjnl-2020-215158 [DOI] [PubMed] [Google Scholar]
- 5. Force U, Krist AH, Davidson KW, et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA 2021;325:962–70. 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 6. Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based 'lung health check' pilot in deprived areas of Manchester. Thorax 2019;74:405–9. 10.1136/thoraxjnl-2017-211377 [DOI] [PubMed] [Google Scholar]
- 7. Goodley P, Balata H, Alonso A, et al. Invitation strategies and participation in a community-based lung cancer screening programme located in areas of high socioeconomic deprivation. Thorax 2023;79:58–67. 10.1136/thorax-2023-220001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lebrett MB, Balata H, Evison M, et al. Analysis of lung cancer risk model (PLCOM2012 and LLPV2) performance in a community-based lung cancer screening programme. Thorax 2020;75:661–8. 10.1136/thoraxjnl-2020-214626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Data and analysis code are available on reasonable request.