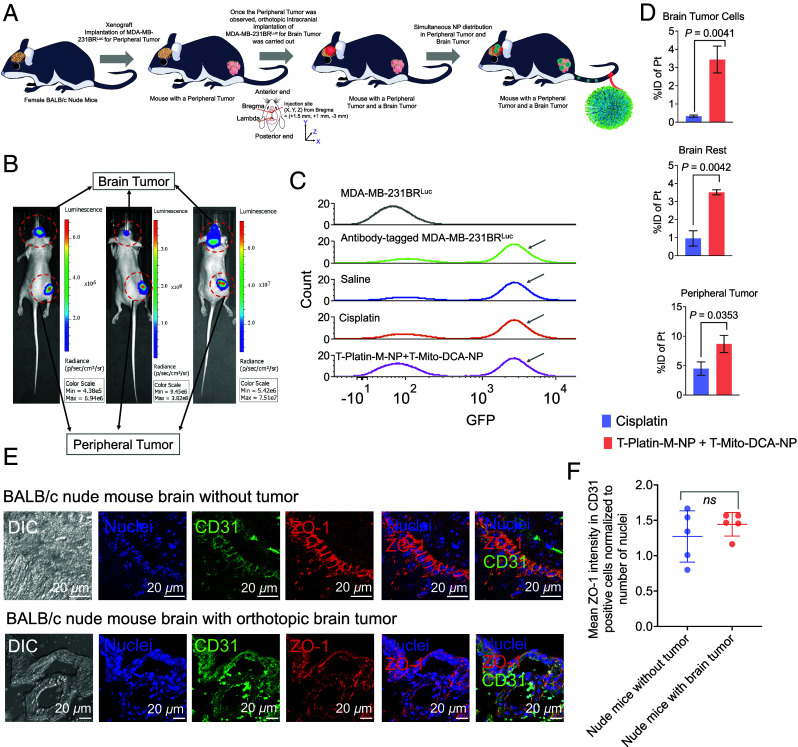
Fig. 5.
Distribution of T-Platin-M-NPs in a dual tumor model. (A) Schematic representation showing the experimental design where biodistribution of T-Platin-M-NPs was studied in female BALB/c nude mice that were inoculated with MDA-MB-231-BRLuc cells subcutaneously at the right flank followed by secondary tumor implantation in the brain through stereotactic intracranial injection. Animals were randomly divided into 3 groups: saline (n = 5), cisplatin (n = 5), and polytherapy (n = 4). Organs were harvested for biodistribution post treatment. Animals in the saline group received 100 µL saline injection, those in the cisplatin group were administered with 5 mg/kg cisplatin, and animals in the polytherapy group received a combination of T-Platin-M-NP at 10 mg/kg dosage with respect to Platin-M and T-Mito-DCA-NP at 30 mg/kg with respect to Mito-DCA. Here, T-Mito-DCA-NP was administered 24 h post T-Platin-M-NP administration. The tail i.v. route was used for administering all the test articles. All major organs were harvested 24 h post the terminal dosage of T-Mito-DCA-NP. (B) Representative IVIS images showing the simultaneous appearance of primary tumor and brain tumor demarcated using red dotted circles. (C) Cell sorting performed using flow cytometry to sort the brain tumor initiating MDA-MB-231-BRLuc cells from the remaining brain cells to quantify the (D) %injected dose of platinum (Pt) in cisplatin- and T-Platin-M-NPs + T-Mito-DCA-NPs-treated mice. Biodistribution was quantified in sorted brain tumor cells, the remaining brain tissue, and in primary tumor. The statistical significance was determined using the unpaired t test and the data was represented as mean ± SD. Sorting of the tumor specific cells from the whole brain tissue was confirmed by using luciferase antibody in the whole brain suspension prior to sorting. For each of the three groups, the cell population which stained positive for luciferase was collected as brain tumor cells for further biodistribution analyses. The residual suspension was used for analyzing biodistribution in the remaining brain tissue. Luciferase-tagged MDA-MB-231-BRLuc cells were used as positive control and unstained cells were used as negative control. (E) Representative images showing the analyses of BBB integrity by using tumor-containing brain via immunofluorescence for tight junction protein ZO-1 in CD31 expressing brain endothelial cells and comparison with nude mice without any tumor confirming that the BBB remains intact after intracranial tumor implantation. (F) Quantification of ZO-1 in CD31 positive brain endothelial cells. Statistical analyses were performed using the unpaired t test.