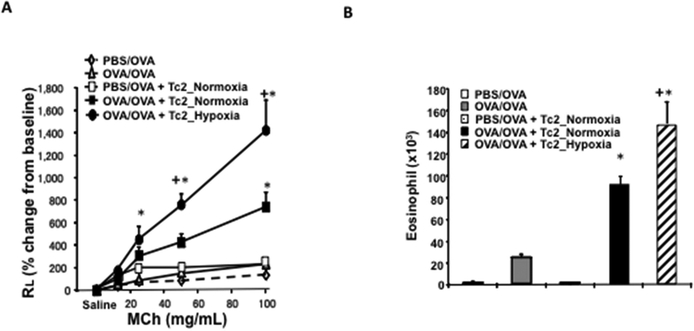
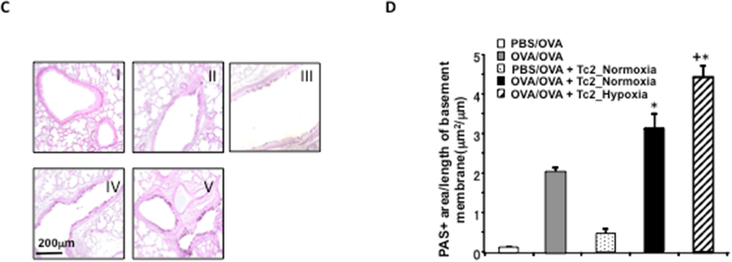
Figure 2: Adoptive transfer of hypoxia-exposed CD8+ Tc2 cells into OVA-sensitized CD8-deficient recipients enhances AHR and airway inflammation.
CD8-deficient mice were initially sensitized and challenged and received no cells (OVA/OVA), 4×105 CD8+ T cells differentiated in IL-2+IL-4 under normoxia (OVA/OVA + Tc2_Normoxia) or 4×105 CD8+ T cells differentiated in IL-2+IL-4 under hypoxia (OVA/OVA + Tc2_Hypoxia) prior to secondary challenge. As control, CD8-deficient recipients were not initially sensitized but challenged and received no cells (PBS/OVA) or 4×105 CD8+ T cells differentiated in IL-2+IL-4 under normoxia (PBS/OVA + Tc2_Normoxia) prior to secondary challenge. (A) Changes in RL were measured in response to increasing concentrations of inhaled MCh. (B) Eosinophil numbers in BALfluid. (C) Representative photomicrographs of lung histology (PAS staining): I) PBS/OVA, II) OVA/OVA, III) PBS/OVA + Tc2 normoxia, IV) OVA/OVA + Tc2 normoxia, and V) OVA/OVA + Tc2 hypoxia. (D) Quantitative analysis of goblet cells. Data for A, B, and D (means±SEM) are from 2 independent experiments with 8 mice/group. *p<0.05 compared to PBS/OVA and OVA/OVA, +p<0.05 compared to OVA/OVA + Tc2 normoxia.