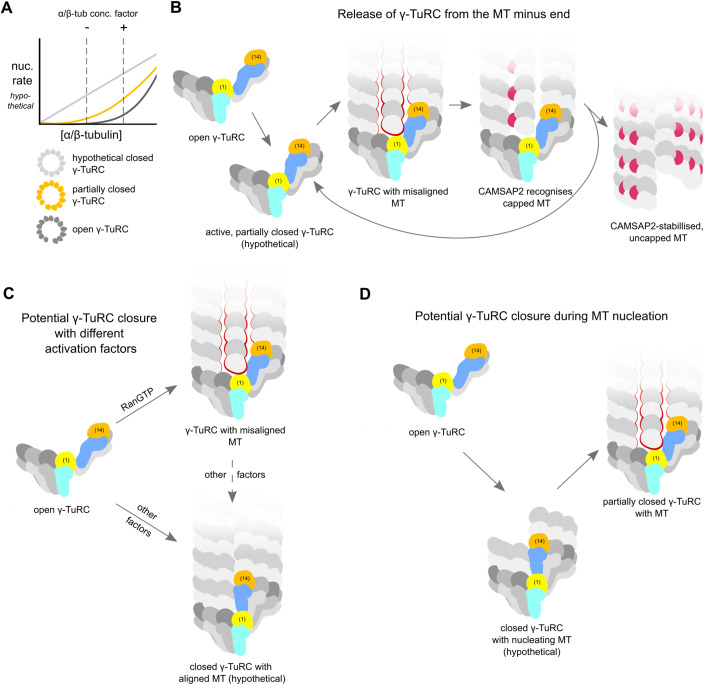
Figure 5. Possible roles for partial γ-TuRC closure and MT misalignment in the regulation of MT nucleation and release.
(A) The open γ-TuRC (dark grey curve) displays highly cooperative nucleation behaviour in response to α/β-tubulin concentration but has a low base rate of nucleation (Thawani et al, 2020). The fully closed γ-TuRC (light grey) has a high base rate of nucleation with a lack of cooperativity. A partially closed γ-TuRC (orange) would likely have an intermediate base rate of nucleation, while still retaining some cooperativity. Such behaviour could aid synergy of the γ-TuRC with α/β-tubulin-enriching factors. Graphs are for illustrative purposes and do not represent experimental or simulated data. (B) Misalignment between the γ-TuRC and MT protofilaments may promote release of freshly nucleated MTs from the γ-TuRC, e.g. by CAMSAP2 (red), and recycling of nucleation-competent γ-TuRC. Spoke 1 and 14 are coloured to highlight different degrees of γ-TuRC closure. Spoke numbers are indicated for spokes 1 and 14. Missing interactions around the protofilament at spoke 2 highlighted with red outlines. (C) Different activation cues may induce different degrees of γ-TuRC closure to accommodate differential requirements of nucleation rate and minus end stability. (D) The γ-TuRC may transiently visit a fully closed conformation that favours MT nucleation and subsequently relax to the partially closed conformation observed in our study to favour MT release.