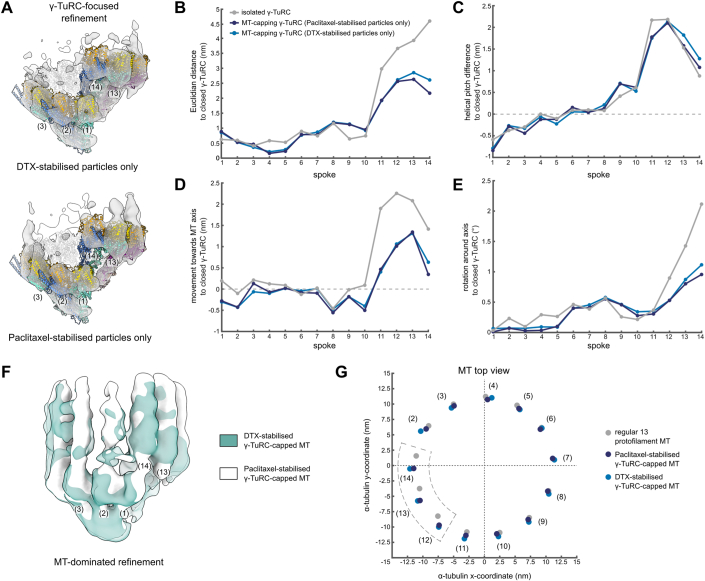
Figure EV3. The conformation of the γ-TuRC and attached MT is not affected by the identity of the MT stabilisation agent.
(A) Reconstruction obtained from γ-TuRC-focused refinement of MT-capping γ-TuRCs with only DTX-stabilised particles (top) or only Paclitaxel-stabilised particles (bottom). A model for the γ-TuRC was generated for each reconstruction separately by spoke-wise rigid body docking. A high confidence threshold is used to highlight the fit at the level of γ-tubulin. Spoke numbering is indicated. Colouring as in Fig. 2A. (B–E) Euclidian translation distance (B), downward change in helical pitch (C), translation towards the MT axis (D) and rotation around the MT axis (E) required to convert the models to the hypothetical, fully closed γ-TuRC for each spoke. Parameters measured from the centre of mass of γ-tubulin. (F) Reconstructions of MT-dominated refinements of γ-TuRC-capped MTs with only DTX-stabilised (green) or only Paclitaxel-stabilised particles (white). Density for spokes in the background was hidden for visual clarity. Colouring scheme is indicated. (G) Coordinates of the centre of mass for the first α-tubulin in each protofilament in the plane orthogonal to the MT axis for regular 13 protofilament lattice (grey), Paclitaxel-stabilised γ-TuRC-capped MT (dark blue) and DTX-stabilised γ-TuRC-capped MT (light blue). Protofilaments are numbered by the respective spokes in the γ-TuRC. The MT axis is placed on the origin, (0,0). Colouring scheme is indicated. Source data are available online for this figure.