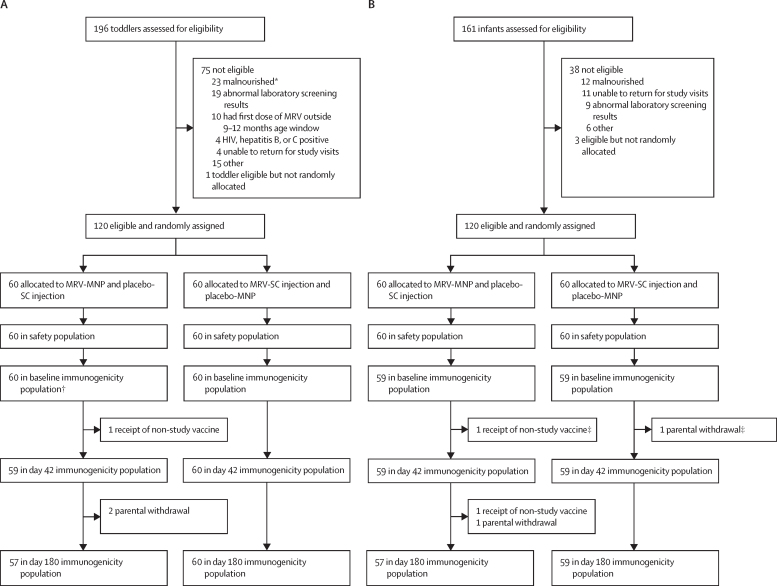
Figure 1.
Trial profile—toddler and infant cohorts
(A) Toddler cohort (B) Infant cohort. MNP=microneedle patch. MRV=measles and rubella vaccine. SC=subcutaneous. *Defined as weight-for-length Z score of <2 SDs below the mean. †In the toddler MRV-MNP group the baseline immunogenicity sample was analysed for the toddler who received a non-study vaccine between baseline and day 42, thus 60 baseline sample results were available. ‡One infant in the MRV-MNP group and one infant in the MRV-SC group were withdrawn between baseline and day 42. The baseline samples for these two infants were not analysed, hence 59 infants were included in the immunogenicity population at baseline as well as day 42.