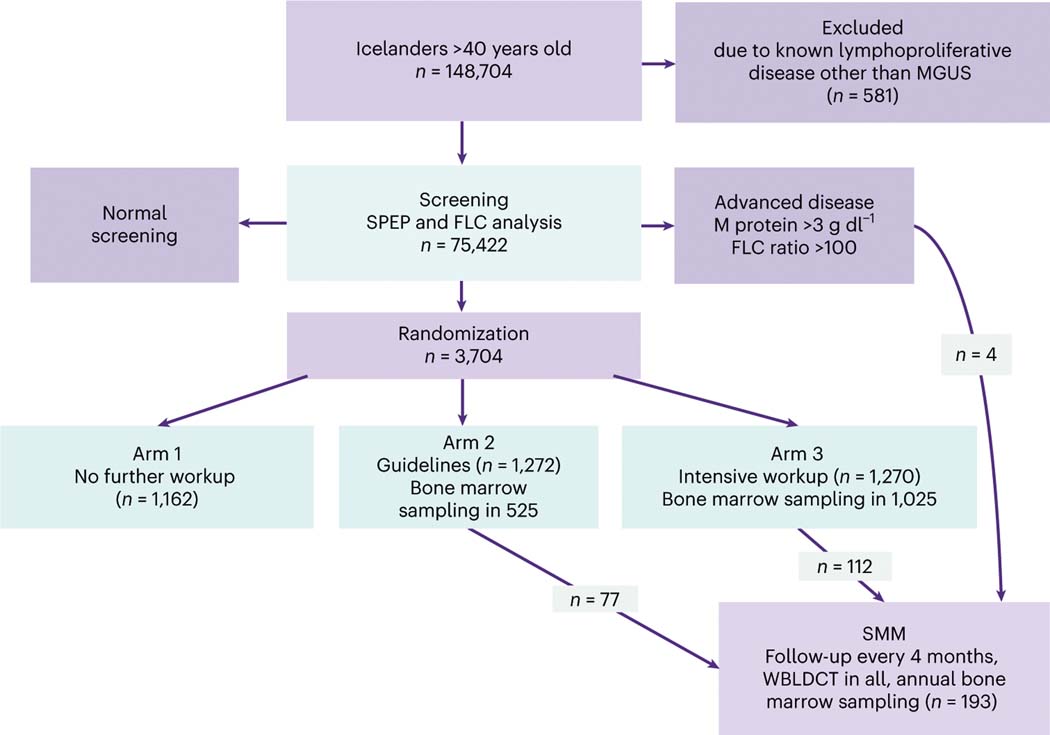
Fig. 1 |. The iStopMM study design.
All Icelanders >40 years old residing in Iceland on 9 September 2016 (148,704 individuals) were invited to participate. The only exclusion criterion was previously known lymphoproliferative disease other than MGUS or SMM. Participants with abnormal screening tests based on a positive M protein result on SPEP or an abnormal FLC analysis entered an RCT. The RCT was divided into three arms. Participants in arm 1 continued care in the Icelandic healthcare system as though they were never screened. Participants in arms 2 and 3 were evaluated at the study clinic for initial assessment and followup, with participants in arm 2 receiving care according to current guidelines6, including bone marrow sampling and skeletal surveys for all except those with low-risk MGUS. In arm 3, bone marrow testing and WBLDCT were offered to all participants. Participants with previously known MGUS were randomized only to arms 2 and 3. Participants with an M protein concentration ≥3.0 g dl−1 or an FLC ratio ≥100 were not randomized but were all called in for evaluation. Participants diagnosed with SMM were enrolled to more intensive follow-up at least every 4 months, with annual bone marrow sampling and WBLDCT. In this article, we report the results from the initial blood and bone marrow screening. The RCT is still ongoing.