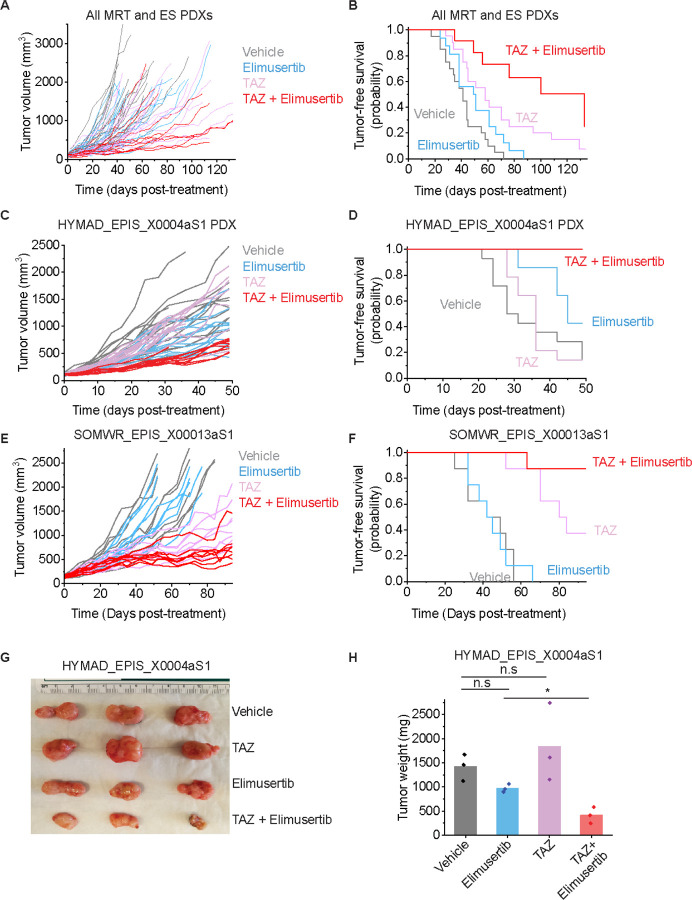
Figure 5: TAZ plus elimusertib improves therapeutic response in vivo:
(A) Tumor growth curves for 5 mouse PDXs treated with the indicated drug regimen. n = 20 mice for vehicle and elimusertib-treated groups, n = 21 for TAZ and TAZ + elimusertib-treated groups. Vardi U-test p = 2.3E-2 and 0.20 for combination vs. elimusertib or TAZ, respectively. (B) Kaplan-Meier curves showing tumor-free survival (defined as tumor volume ≤ 1,000 mm3) for the PDXs in panel C. Mean survival is 51 days (95% CI: 42–60 days) for elimusertib, 68 days (95% CI: 53–82 days) for TAZ to 100 days (95% CI: 74–124 days) for the combination. Log-rank test p = 5.8E-4 and 3.8E-2 for combination versus elimusertib or TAZ, respectively. (C) Tumor growth curves for the HYMAD_EPIS_X0004aS1 PDX model treated with the indicated drug regimen. Vardi U-test p = 2.0E-4 for combination versus elimusertib or TAZ. n = 14 mice per treatment group. (D) Kaplan-Meier curves showing tumor-free survival (defined as tumor volume ≤ 1,000 mm3) for the PDXs in panel C. Log-rank test p = 6.2E-3 and 6.3E-5 for combination versus elimusertib or TAZ, respectively. (E) Tumor growth curves for the SOMWR_EPIS_X00013aS1 PDX model. Vardi U-test p = 1.0E-3 and 6.0E-2 for combination versus elimusertib and TAZ, respectively. n = 8 mice for all groups. (F) Kaplan-Meier curves showing tumor-free survival (defined as tumor volume ≤ 1,000 mm3) for the SOMWR_EPIS_X00013aS1 PDX model; Mean survival is 90 days (95% CI: 83–97 days) for combination versus 80 days (95% CI: 70–90 days) for TAZ and 45 days (95% CI: 37–52 days) for elimusertib. Log-rank test p = 9.5E-5 and 6.0E-2 for combination versus elimusertib and TAZ, respectively. (G) Image of representative tumors extracted from mice in C-D and on Day 52 of treatment (H) their corresponding weights. *p = 6.7E-3 by two-sided Student’s t-test.