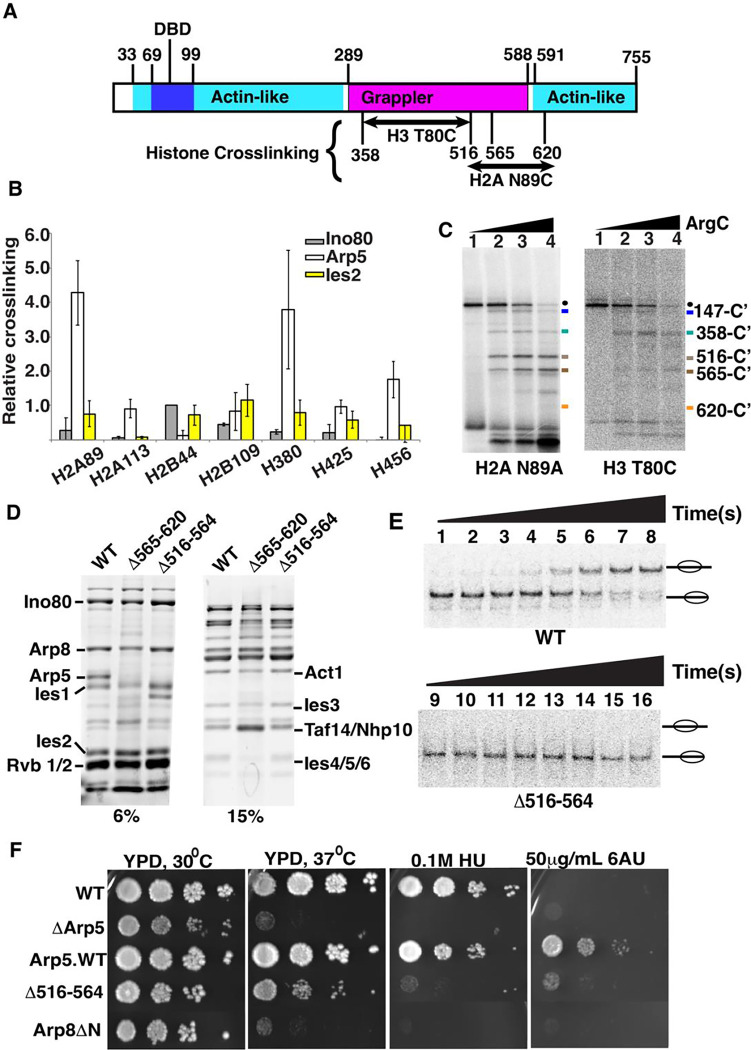
Figure 1. Two regions of Arp5’s grappler domain associate with the acid pocket of nucleosomes.
(A). The domain organization is shown for the Arp5 subunit of INO80 with the actin-like portion highlighted in cyan and the grappler domain in purple. The DNA binding domain of Arp5 is indicate (dark blue. The Arp5 regions crosslinked to residue 80 of histone H3 and residue 89 of H2A are labeled. (B) The interactions of Arp5 with the histone octamer face of nucleosomes are probed by site-specific histone crosslinking. The relative efficiency of crosslinking Arp5, Ies2 and Ies6 is shown for three replicates. Error bars represent the mean ± SD.(C) The regions of Arp5 crosslinked to histones are mapped by immobilizing the C-terminus of Arp5 and partial digesting with Arg-C. Labeled proteolytic fragments of Arp5 are separated on a 4–20% SDS-PAGE and visualized by phosphorimaging. (D) The two regions are individually deleted, and the complex purified using by FLAG affinity chromatography. (E) Nucleosome mobilizing activity of the INO80 complex with Arp5 Δ516–564 (lanes 1–8) is compared to wild type INO80 (lanes 9–16) using EMSA. (24nM) of nucleosomes were saturated with 25nM of INO80 complex (WT or mutant) with addition of 80 μM ATP. (F) The phenotype of the yeast strain with Arp5 Δ516–564 was compared to wild type Arp5 (WT, Arp5 WT), N-terminal truncation of Arp8 (Arp8 ΔN) and the absence of Arp5 (ΔArp5).