Abstract
Background
Isatuximab, an anti-CD38 antibody, has been widely used in treatments for patients with relapsed/refractory multiple myeloma (MM). Despite its high efficacy, not all patients achieve a lasting therapeutic response with isatuximab.
Objective
We tried to identify biomarkers to predict the effectiveness of isatuximab by focusing on the host's immune status before treatment.
Methods
We retrospectively analyzed the cases of 134 relapsed/refractory MM patients in the Kansai Myeloma Forum database who had received only a first isatuximab treatment.
Results
Among the 134 patients, an isatuximab, pomalidomide and dexamethasone (Isa-PD) regimen, isatuximab, carfilzomib and dexamethasone (Isa-KD) regimen and isatuximab and/or dexamethasone (Isa-D) regimen were used in 112, 15 and 7 patients, respectively. The median age at treatment, number of prior treatment regimens, and progression-free survival (PFS) were 71, 6, and 6.54 months, respectively. Multivariate analysis showed that the PFS under the Isa-PD regimen was longer in patients with higher lymphocyte/monocyte ratio (LMR ≥ 4), fewer prior treatment regimens (< 6), and no use of prior daratumumab treatment. The OS under the Isa-PD regimen was longer in patients with higher white blood cell counts (WBC counts ≥ 3000/μL) and higher LMR. The PFS under the Isa-D regimen was longer in patients with fewer prior treatment regimens in univariate analysis, but no parameters were correlated with PFS/OS under the Isa-KD regimen.
Conclusion
We found that the patients with higher LMR (≥ 4) could obtain longer PFS and OS under the Isa-PD regimen. Other cohort studies of isatuximab treatment might be necessary to substantiate our results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03711-8.
Keywords: Multiple myeloma, Isatuximab, Lymphocyte/monocyte ratio, Predictive markers
Introduction
The prognosis of multiple myeloma (MM) patients has been dramatically improved by proteasome inhibitors, immunomodulatory drugs, and anti-CD38 antibodies [1–5]. Among them, isatuximab, one of the new anti-CD38 antibodies, has shown a high response rate with a superior prognosis for relapsed/refractory MM patients when used in combination with pomalidomide, carfilzomib and dexamethasone [3–5]. However, although 60% to 80% of MM patients responded to isatuximab treatment in clinical trials [3–5], some patients did not benefit sufficiently from isatuximab treatment, and a certain number of patients could not obtain a therapeutic response. Although immunotherapies are increasingly playing a role in MM treatment [6], we do not have any appropriate specific biomarkers that could predict the response or the durable efficacy of immunotherapies such as isatuximab before treatment [7].
To identify patients who might benefit from isatuximab before treatment, we focused on the immunological aspect of isatuximab. The mechanisms of action of isatuximab are immune-mediated effects, such as complement- or antibody-dependent cell-mediated cytotoxic effects, antibody-dependent cell phagocytic activity, depletion of CD38-positive regulatory immune cells, and direct killing activity of antibodies against myeloma cells [8–11]. Previously, we reported that pretreatment lymphocyte or monocyte counts could predict the efficacy of other antibodies used for the treatment of MM, such as elotuzumab and daratumumab [12, 13], or bispecific T-cell engager antibody [14]. Also, several studies have indicated that the lymphocyte/monocyte ratio (LMR) could predict the prognosis of MM patients [15, 16]. Here, we hypothesized that the immune conditions (as represented by white blood cell counts LMR, and neutrophil/lymphocyte ratio) before isatuximab treatment might predict its efficacy. As a proof of concept, we conducted a retrospective observational analysis using real-world data from the Kansai Myeloma Forum (KMF) database in Japan.
Methods
Study design and participants
KMF, a study group consisting of 131 physicians in 43 facilities in Japan, established a database that includes physician-reviewed, real-world clinical data on the diagnosis, treatment, and periodical follow-up of patients with plasma cell dyscrasias. This study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (approval no. R2887). A total of 4,814 patients with plasma cell dyscrasias were registered in the KMF database in September 2023. All these patients were diagnosed as having MM or MM-related disorders based on institutional assessment. For purposes of the present retrospective analysis, we selected the patients who were older than 20 years, had relapsed MM, and had been treated with an isatuximab-containing regimen between August 2020 and August 2023 (after its June 2020 approval for clinical use in Japan). A total of 112 patients administered a total of 128 isatuximab treatments met the above criteria (Supplementary Fig. 1). We selected the patients who had received only a first administration of isatuximab and omitted the 14 patients with a second and 2 patients with a third isatuximab treatment. We thus analyzed 112 relapsed MM patients who had undergone only first isatuximab treatment and were followed until October 2023.
The patients' responses to treatment were assessed based on the international uniform response criteria [17] for multiple myeloma. The patients' best responses against isatuximab were classified by institutional physicians into five categories: complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD), and progressive disease (PD). For the high-risk cytogenetic abnormalities, we adopted the abnormalities reported in the International Myeloma Working group consensus statement [18], such as deletion 17p, t(4;14) and t(14;16). Unfavorable cytogenetic abnormalities were categorized by a fluorescence in situ hybridization (FISH) analysis. CD138 purification was not performed for FISH analysis, and patients with 20% positive cells were considered positive for FISH analysis.
Statistical methods
We calculated the progression-free survival (PFS) for isatuximab treatment as the time from isatuximab treatment until the date of progression of MM, death by any cause or the date of last contact as a primary endpoint. The data were censored for the date of last administration of isatuximab in cases with planned isatuximab cessation. The laboratory data 1–7 days before cycle 1 day 1 isatuximab treatment after the previous treatment were used. We determined the cutoff values using the 25th, 50th and 75th percentile values (Supplementary Fig. 2A-E and 3) with reference to the previous studies [12–16]. We set the secondary endpoint as overall survival (OS).
The survival curves based on the PFS and OS curve were plotted using the Kaplan–Meier method, and the log-rank test was used for comparisons among groups. The Cox proportional hazard model was used to calculate the hazard ratio for each variable along with the 95% confidence interval (CI). All the variables were applied in the univariate analysis and a multivariate analysis was conducted for the variables which showed a p value of less than 0.1 in the univariate analysis. We used the bootstrap method [19, 20] to validate our multivariate analysis results for the variables that showed a p value of less than 0.1 in the univariate analysis (Table 3). In each step, 1000 bootstrap samples with replacements were created from the dataset [19, 20]. All statistical analyses were performed using the EZR (ver. 1.61) software package (Saitama Medical Center/Jichi Medical University, Saitama, Japan) [21] along with a graphical user interface for the R software package (ver. 4.2.2; The R Foundation for Statistical Computing) or SPSS software ver. 29.02 (IBM, USA). P-values < 0.05 were considered significant in all analyses.
Table 3.
Multivariate analyses of PFS and OS under the Isa-PD regimen
| Factors | PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | P-value* | Hazard ratio | 95% CI | P-value | P-value* | ||
| White blood cell counts | ≥ 3000/μl | 1 | 1 | ||||||
| < 3000/μl | 1.721 | 0.970–3.056 | 0.064 | 0.081 | 2.990 | 1.627–5.493 | < 0.001 | 0.002 | |
| Lymphocyte/monocyte ratio | ≥ 4 | 1 | 1 | ||||||
| < 4 | 2.494 | 1.216–5.116 | 0.013 | 0.009 | 5.188 | 1.840–14.630 | 0.002 | < 0.001 | |
| Neutrophil/lymphocyte ratio | < 2.3 | 1 | 1 | ||||||
| ≥ 2.3 | 1.409 | 0.790–3.907 | 0.231 | 1.467 | 0.782–2.751 | 0.231 | |||
| κ/λ ratio | 0.1–10 | 1 | 1 | ||||||
| ≤ 0.1, ≥ 10 | 1.058 | 0.502–2.228 | 0.883 | 1.116 | 0.468–2.659 | 0.805 | |||
| NA | 1.181 | 0.442–3.159 | 0.740 | 0.916 | 0.271–3.100 | 0.887 | |||
| B2MG | < 3.5 mg/L | 1 | 1 | ||||||
| ≥ 3.5 mg/L | 1.927 | 0.849–4.375 | 0.117 | 1.512 | 0.601–3.809 | 0.380 | |||
| NA | 1.837 | 0.876–3.850 | 0.107 | 1.357 | 0.579–3.182 | 0.107 | |||
| Prior regimen numbers | < 6 | 1 | 1 | ||||||
| ≥ 6 | 2.177 | 1.214–3.901 | 0.009 | 0.012 | 1.838 | 0.897–3.768 | 0.097 | 0.065 | |
| Prior use of daratumumab | No | 1 | 1 | ||||||
| Yes | 2.458 | 1.357–4.453 | 0.003 | 0.011 | 2.014 | 1.032–3.929 | 0.040 | 0.062 | |
Multivariate analyses of PFS and OS under the Isa-PD regimen were performed using the factors that showed P < 0.1 in univariate analysis. The Cox proportional hazard model was used to calculate the hazard ratio for each variable; the 95% CI and p-value are shown. *The P-value after the bootstrapping process (1000 samples) using the factors that showed P < 0.1 in multivariate analysis
PFS progression-free survival, OS overall survival, CI confidence interval, B2MG β2 microglobulin
Results
Progression-free survival of isatuximab in relapsed multiple myeloma
The characteristics of the patients undergoing each regimen are summarized in Table 1 and Supplementary Table 1. In brief, a total of 134 patients who were treated with isatuximab for the first time were analyzed. The median age at the time of isatuximab treatment was 71 (range: 34–87) years old. The numbers of patients treated with the Isa-PD, Isa-KD and Isa-D regimens were 112 (83.6%), 15 (11.2%) and 7 (5.2%), respectively. The median number of prior regimens was 6 and daratumumab had been used before isatuximab treatment in 84 cases (63.6%): 69 cases with an Isa-PD regimen, 13 cases with an Isa-KD regimen and 2 cases with an Isa-D regimen. The histograms of laboratory data before isatuximab treatment are shown in Supplementary Fig. 2. Patients with a CR, VGPR or PR were regarded as having a therapeutic response to isatuximab; these included 44 patients (39.3%) treated with the Isa-PD regimen, 5 cases (33.3%) treated with the Isa-KD regimen and 1 case (16.7%) treated with the Isa-D regimen (Supplementary Fig. 3).
Table 1.
Patient characteristics for the whole cohort
| Type of treatment regimen | ||
|---|---|---|
| Isa-PD | 112 (85.6%) | |
| Isa-KD | 15 (11.2%) | |
| Isa-D | 7 (5.2%) | |
| Age (years) at treatment | ||
| Median (range) | 71 (34–87) | |
| Gender | ||
| Male | 71 (54.2%) | |
| Female | 60 (45.8%) | |
| Type of heavy chain | ||
| IgG | 81 (60.9%) | |
| IgA | 25 (18.8%) | |
| BJP | 21 (15.8%) | |
| IgM | 1 (0.8%) | |
| IgD | 3 (2.3%) | |
| NA | 2 (1.5%) | |
| Type of light chain | ||
| λ | 87 (64.9%) | |
| κ | 44 (32.8%) | |
| NA | 3 (2.2%) | |
| ISS stage at diagnosis | ||
| I | 42 (31.3%) | |
| II | 42 (31.3%) | |
| III | 39 (21.6%) | |
| NA | 21 (15.7%) | |
| High-risk cytogenic abnormality | ||
| None | 58 (43.3%) | |
| At least one | 47 (35.1%) | |
| NA | 29 (21.6%) | |
| Laboratory data before isatuximab treatment | ||
| White blood cell count | (/μL, median, range) | 4580 (1240–13230) |
| Lymphocyte/monocyte ratio | (median, range) | 2.36 (0.05–193.0) |
| Neutrophil/lymphocyte ratio | (median, range) | 2.30 (0.03–67.0) |
| Free light chain | (mg/L, median, range) | |
| κ | 34.3 (0.5–12,040) | |
| λ | 10.1 (0.4–4020) | |
| κ/λ ratio | 3.1 (0.001–4089) | |
| B2MG | (mg/L, median, range) | 2.7 (0.17–14.34) |
| Prior regimen numbers | ||
| Median (range) | 6 (2–18) | |
| Prior use of daratumumab | ||
| Yes | 84 (63.6%) | |
| Follow-up period of survivor | ||
| Median days (range) | 472 (7–1137) | |
The characteristics of multiple myeloma patients treated with isatuximab regimens are shown in Table 1. Laboratory data were collected before the isatuximab treatment
NA not available, ISS International Staging system; β2 microglobulin: B2MG
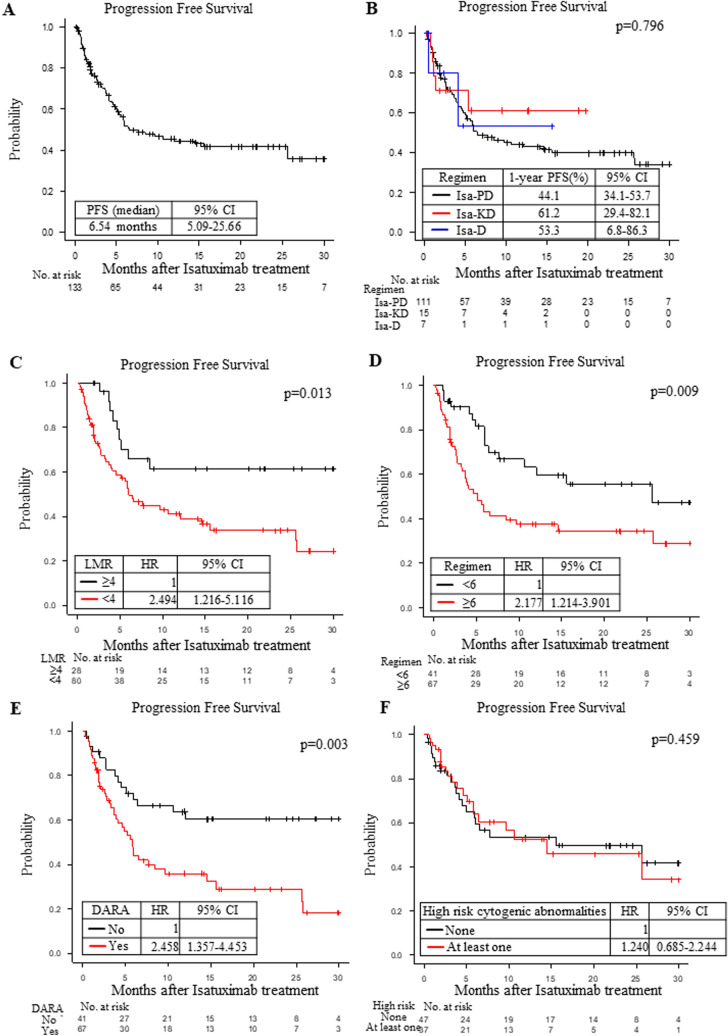
The median PFS under isatuximab treatment was 6.54 (95%CI: 5.09–25.66) months in this cohort (Fig. 1A). When we compared the PFS by the regimens, the 1-year PFS ratios under the Isa-PD, Isa-KD and Isa-D regimens were 44.1% (34.1–53.7), 61.2% (29.4–82.1) and 53.3% (6.8–86.3), respectively (Fig. 1B; not significant). The median OS under isatuximab treatment was 26.4 (95%CI: 15.97-Not available) months in this cohort (Fig. 2A).
Fig. 1.
A The progression-free survival (PFS) of the multiple myeloma (MM) patients treated with isatuximab. The entire cohort was used to calculate PFS. Median PFS (months) values with the 95% CI (confidence interval) are shown. B The PFS of the MM patients under the following regimens: isatuximab, pomalidomide and dexamethasone (Isa-PD, black); isatuximab, carfilzomib and dexamethasone (Isa-KD, red); and isatuximab and dexamethasone (Isa-D, blue). One-year PFS values (%) with the 95% CI are shown. C The PFS of the MM patients under the Isa-PD regimen according to the lymphocyte/monocyte ratio (LMR): 4 or more (black) or less than 4 (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. D The PFS of the MM patients under the Isa-PD regimen according to the number of previous regimens: 6 or more (black) or less than 6 (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. E The PFS of the MM patients under the Isa-PD regimen according to the prior use of daratumumab (DARA): No (black) or Yes (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. F The PFS of the MM patients under the Isa-PD regimen according to the high risk cytogenic abnormalities: none (black) or at least one (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. The number of patients at risk in each group is shown in the lower panel of each figure
Fig. 2.
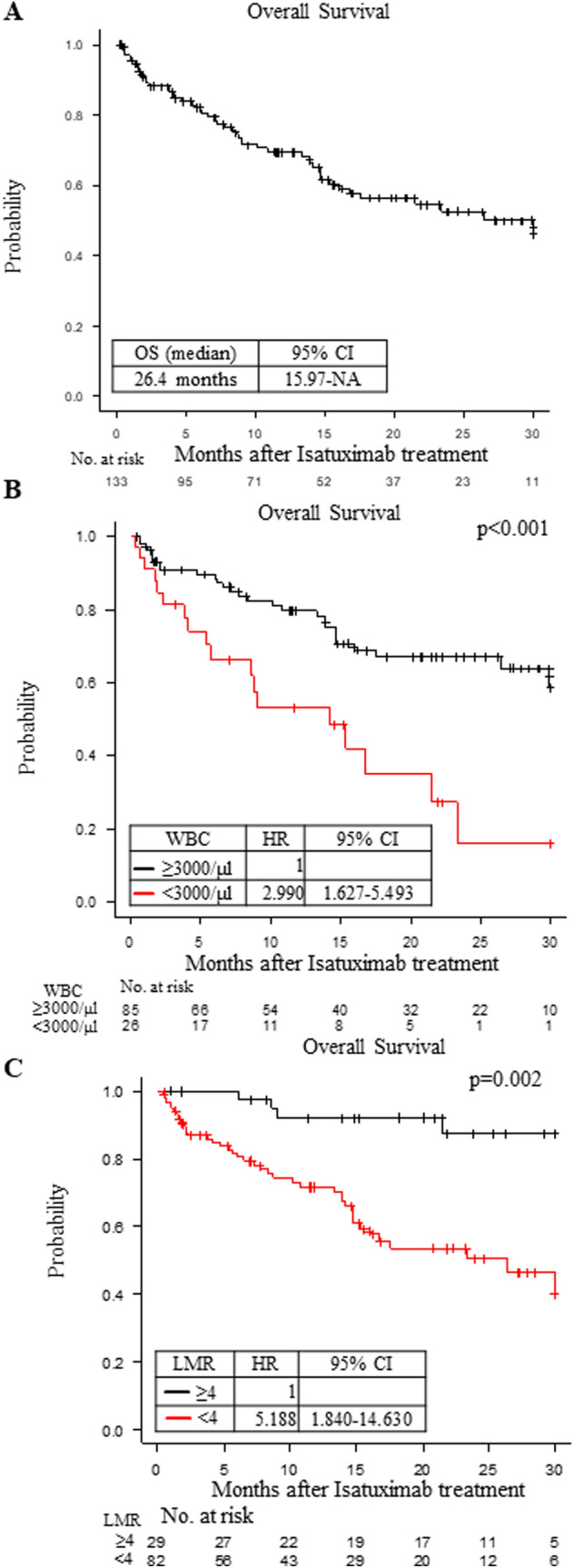
A The overall survival (OS) of the MM patients for the entire cohort. Median OS (months) with the 95% CI is shown. B The overall survival (OS) of the MM patients under the Isa-PD regimen according to the white blood cell (WBC) counts: 3000/μl or more (black) or less than 3000/μl (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. C The OS of the MM patients under the Isa-PD regimen according to the lymphocyte/monocyte ratio (LMR): 4 or more (black) or less than 4 (red). The hazard ratio (HR) with the 95% CI is shown. The survival curves were adjusted by the significant factors in the multivariate analysis. The number of patients at risk in each group is shown in the lower panel of each figure
Underlying factors affecting the PFS and OS under the Isa-PD regimen
We analyzed the underlying factors affecting the PFS under each treatment regimen. We found that the PFS of patients under the Isa-PD regimen was longer in those with higher white blood cell (WBC) count (≥ 3000/μl; p = 0.048), higher LMR (≥ 4; p = 0.002), lower neutrophil/lymphocyte ratio (NLR < 2.3; p = 0.020), lower β2 microglobulin level (B2MG < 3.5 mg/L; p = 0.009), and lower prior regimen number (< 6; p = 0.013) and in those not using daratumumab prior to isatuximab treatment (p = 0.041; Table 2 and Fig. 1C-F). The PFS of patients under the Isa-D regimen was longer in those with a lower prior regimen number (< 6; p = 0.046, Supplementary Table 2), but we could not find any factors which correlated to PFS under the Isa-KD regimen (Supplementary Table 2).
Table 2.
Univariate analysis for progression-free survival and overall survival under the Isa-PD regimen
| Univariate analysis | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | 1-year-PFS (%) | 95% CI | Hazard ratio | 95% CI | P-value | 1-year-OS (%) | 95% CI | Hazard ratio | 95% CI | P-value | |
| Age | < 65 years | 41.4 | 22.8–59.1 | 1 | 0.906 | 76.4 | 56.8–88.0 | 1 | 0.434 | ||
| ≥ 65 years | 43.4 | 31.6–54.7 | 0.967 | 0.556–1.682 | 65.5 | 52.9–75.4 | 1.301 | 0.671–2.523 | |||
| Gender | Male | 38.4 | 25.2–51.5 | 1 | 0.144 | 69.0 | 54.3–79.8 | 1 | 0.607 | ||
| Female | 50.2 | 35.2–63.6 | 0.685 | 0.411–1.143 | 69.4 | 54.4–80.4 | 0.859 | 0.481–1.535 | |||
| High risk cytogenic abnormalities | None | 51.7 | 35.4–65.8 | 1 | 0.280 | 76.2 | 60.1–86.5 | 1 | 0.674 | ||
| At least one | 42.5 | 25.6–58.4 | 1.240 | 0.685–2.244 | 67.1 | 49.2–79.9 | 1.338 | 0.695–2.579 | |||
| NA | 33.3 | 15.9–51.9 | 1.667 | 0.884–3.144 | 60.0 | 36.8–77.0 | 1.232 | 0.564–2.693 | |||
| White blood cell counts | ≥ 3000/μl | 49.4 | 37.5–60.3 | 1 | 0.048 | 76.3 | 65.0–84.4 | 1 | < 0.001 | ||
| < 3000/μl | 27.9 | 12.1–46.2 | 1.732 | 0.999–3.011 | 47.7 | 27.3–65.6 | 2.824 | 1.551–5.143 | |||
| Lymphocyte/monocyte ratio | ≥ 4 | 63.9 | 42.0–79.3 | 1 | 0.002 | 88.3 | 67.9–96.1 | 1 | < 0.001 | ||
| < 4 | 37.1 | 26.1–48.2 | 2.960 | 1.455–6.023 | 62.3 | 50.1–72.3 | 5.523 | 1.975–15.440 | |||
| Neutrophile/lymphocyte ratio | < 2.3 | 54.6 | 39.9–67.1 | 1 | 0.020 | 78.7 | 64.8–87.6 | 1 | 0.011 | ||
| ≥ 2.3 | 32.6 | 19.6–46.3 | 1.822 | 1.089–3.048 | 59.3 | 44.1–71.7 | 2.152 | 1.177–3.936 | |||
| ISS stage | I | 42.8 | 25.9–58.7 | 1 | 0.935 | 69.3 | 50.2–82.2 | 1 | 0.832 | ||
| II | 44.2 | 25.3–61.6 | 1.764 | 0.720–4.318 | 62.9 | 42.8–77.6 | 2.864 | 0.880–9.315 | |||
| III | 47.9 | 29.3–64.3 | 3.402 | 1.364–8.484 | 67.0 | 47.1–80.8 | 7.153 | 2.297–22.270 | |||
| κ/λ ratio | 0.1–10 | 58.6 | 36.4–75.4 | 1 | 0.074 | 81.2 | 60.4–91.7 | 1 | 0.072 | ||
| ≤ 0.1, ≥ 10 | 38.3 | 26.1–50.4 | 1.783 | 0.937–3.396 | 64.3 | 50.9–75.0 | 1.993 | 0.920–4.317 | |||
| B2MG | < 3.5 mg/L | 59.4 | 38.7–75.2 | 1 | 0.009 | 82.1 | 62.3–92.1 | 1 | 0.007 | ||
| ≥ 3.5 mg/L | 39.4 | 22.1–56.4 | 2.636 | 1.270–5.471 | 60.4 | 40.8–75.3 | 3.113 | 1.347–7.190 | |||
| Prior regimen numbers | < 6 | 62.6 | 44.1–76.5 | 1 | 0.003 | 82.6 | 65.2–91.9 | 1 | 0.006 | ||
| ≥ 6 | 33.9 | 22.5–45.6 | 2.326 | 1.310–4.129 | 60.5 | 47.3–71.4 | 2.581 | 1.280–5.203 | |||
| Prior use of daratumumab | No | 62.2 | 44.5–75.7 | 1 | 0.003 | 78.7 | 61.8–88.8 | 1 | 0.026 | ||
| Yes | 33.9 | 22.2–45.9 | 2.378 | 1.322–4.278 | 63.1 | 49.7–73.8 | 2.089 | 1.078–4.046 | |||
Progression-free survival (PFS) was calculated from the time of isatuximab treatment to the progression of the disease. Overall survival (OS) was calculated from the time of isatuximab treatment to the time of death by any cause. Univariate analyses against PFS and OS under the Isa-PD regimen were performed for each factor. The log-rank test was used for comparisons among groups. One-year-PFS (%) with the 95% confidence interval (CI), hazard ratio with the 95% CI and P-value are shown
PFS progression-free survival, OS overall survival, CI confidence interval, ISS International Staging System, B2MG β2 microglobulin, NA not available
We next analyzed the underlying factors affecting the OS under each treatment regimen. We found that the OS of patients under the Isa-PD regimen was longer in those with higher white blood cell (WBC) counts (≥ 3000/μl, p < 0.001), higher LMR (≥ 4; p < 0.001), lower NLR (< 2.3; p = 0.011), lower B2MG(< 3.5 mg/L; p = 0.007), and lower prior regimen number (< 6; p = 0.006) and in those not using daratumumab prior to isatuximab treatment (p = 0.026; Table 2). We could not find any factors that were correlated with OS under the Isa-KD and Isa-D regimens (Supplementary Table 3).
Higher LMR was correlated with both better PFS and better OS under the Isa-PD regimen
We performed a multivariate analysis of the PFS in patients undergoing the Isa-PD regimen by analyzing all factors that had p values less than 0.1 in the univariate analysis (Table 3). We found that higher LMR (≥ 4; p = 0.013), lower prior regimen number (< 6; p = 0.009) and not using daratumumab prior to isatuximab treatment (p = 0.003) were associated with significantly superior PFS under the Isa-PD regimen (Table 3), and these results were confirmed by the bootstrap method (Table 3). The PFS values of patients undergoing the Isa- PD regimen are shown according to LMR, prior regimen number and prior use of daratumumab in Fig. 1C, D and E.
In multivariate analysis for the OS in patients undergoing the Isa-PD regimen, higher WBC counts (≥ 3000/μl; p < 0.001), higher LMR (≥ 4; p = 0.002) and not using daratumumab prior to isatuximab treatment (p = 0.040) were associated with significantly superior OS (Table 3). The results of higher WBC counts and higher LMR were also confirmed by the bootstrap method (Table 3). The OS in the full cohort of patients undergoing the Isa-PD regimen are shown according to WBC counts and LMR in Fig. 2B and C. The PFS or OS under Isa-KD and Isa-D did not change according to LMR (Supplementary Table 2–3 and Supplementary Fig A-B).
The influence of prior use of daratumumab on treatment with the Isa-PD regimen
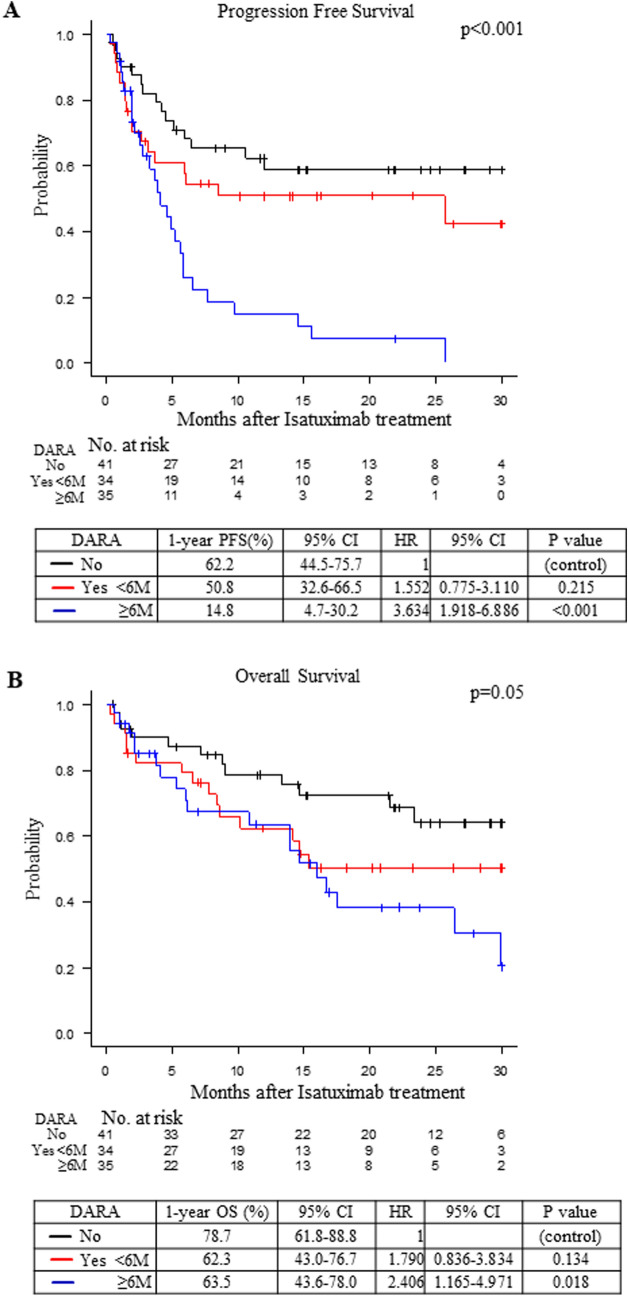
Because the prior use of daratumumab had a negative impact on prognosis after the Isa-PD regimen, we analyzed the interval between last daratumumab treatment and the Isa-PD regimen (≥ 6 months or < 6 months). The PFS and OS under the Isa-PD regimen were significantly shorter in patients who had received the Isa-PD regimen 6 months or more after the last daratumumab treatment than in the patients without prior use of daratumumab (p < 0.001 and p = 0.018, respectively; Fig. 3A-B). However, the PFS and OS under the Isa-PD regimen were not significantly different between patients for whom the Isa-PD regimen was used less than 6 months after the last daratumumab treatment and patients without prior use of daratumumab (not significant, Fig. 3A-B).
Fig. 3.
A The PFS of the MM patients under the Isa-PD regimen according to prior use of DARA: No (black); Yes but treated with isatuximab less than 6 months after the last DARA treatment (red); and Yes but treated with isatuximab 6 months or later after the last DARA treatment (blue). One-year PFS (%) with the 95% CI, the HR with 95% CI, and the p-value are shown. B The OS of the MM patients under the Isa-PD regimen according to prior use of DARA: No (black); Yes but treated with isatuximab less than 6 months after the last DARA treatment (red); and Yes but treated with isatuximab 6 months or later after the last DARA treatment (blue). One-year PFS (%) with the 95% CI, the HR with 95% CI, and the p-value are shown. The number of patients at risk in each group is shown in the lower panel of each figure
Discussion
The effectiveness of immunotherapies against MM could be influenced by the immune status of the host, but there is a lack of useful biomarkers to predict the clinical response before treatment [6, 7]. We have previously reported that the efficacy of elotuzumab and daratumumab could be predicted by lymphocyte and monocyte counts, respectively [12, 13]. Also, several studies have indicated that LMR might predict the prognosis of MM patients [15, 16]. In this study, we demonstrated that LMR (≥ 4) easily predicted the longer PFS and OS of the Isa-PD regimen in relapsed/refractory MM patients. This result was consistent with previous reports [15, 16].
We also found that the patients with lower prior regimen number (< 6) and not using daratumumab prior to isatuximab treatment showed longer PFS under the Isa-Pd regimen. It is not surprising that the PFS under the Isa-PD regimen would be shorter in heavily treated patients with treatment-resistant MM, as fewer prior regimens has been associated with better prognosis in a clinical study [3–5]. We demonstrated that patients with fewer than 6 prior treatment regimens showed significantly longer PFS under the Isa-D regimen, but not under the Isa-KD regimen. This might be due to the limited sample size; we thus considered that the results for the Isa-KD and Isa-D regimens were only exploratory in nature. Also, we showed that the effectiveness of isatuximab was attenuated by the prior use of daratumumab treatment. It has been reported that the number of immune cells, such as NK cells, could be decreased by the use of daratumumab treatment [22–27]. The elimination of myeloma cells by isatuximab depends not only on a direct antibody effect but also on a complement- or antibody-dependent cell-mediated cytotoxic effect [8–11]. Therefore, the prior use of daratumumab might attenuate the effectiveness of isatuximab. As higher WBC counts were correlated with superior OS under the Isa-PD regimen, another explanation could be that higher LMR and WBC counts might be a prerequisite along with a higher number of immune cells; this should be confirmed in future studies. We also considered that the prior treatment to the isatuximab treatment might have influenced WBC counts or LMR. However, we could not find a correlation between the number of prior regimens and WBC counts or LMR (Supplementary Fig. 6A-B). The PFS under the Isa-PD regimen was shorter in patients who had received the Isa-PD regimen 6 months or more after their last daratumumab treatment than in patients who had received the Isa-PD regimen less than 6 months after their last daratumumab treatment (Fig. 3A). Because the choice of treatment depended on individual physicians and the OS was not significantly different between the two groups (Fig. 3B), we speculated that the remaining sensitivity against the anti-CD38 antibody might have differed between the two groups. Although the prior use of daratumumab had a negative impact on PFS under the Isa-PD regimen, simply extending the interval between the last daratumumab administration and the next isatuximab treatment did not appear to restore the efficacy of isatuximab.
Despite these results, we should underscore that isatuximab treatment remains a high priority treatment option for all MM patients due to its high response rate [3–5], even for the relapsed/refractory low LMR patients, who might have a suppressed immune status. Once patients relapse or become refractory to treatments, the effective duration of the next treatments could become shorter, and their prognosis would be much worse [28]. Utilizing the LMR before treatment with an Isa-PD regimen might provide two important pieces of information. First, patients with LMR < 4 have a suppressed immune status and might experience attenuated or unsustained efficacy of isatuximab. Second, the physicians of patients with a suppressed immune status (LMR < 4) might need to prepare for the next treatment after the Isa-PD regimen. Because this was an observational study, we could not tell whether we could change the prognosis of the patients by choosing a treatment other than the Isa-PD regimen. We speculate that LMR is not a prognostic marker in general but a biomarker for the Isa-PD regimen because LMR was difficult to apply for either the Isa-KD or Isa-D regimen in this study. However, this might have been due to the small sample size of patients on the Isa-KD and Isa-D regimen, which needs to be verified by other datasets.
There were several limitations in this study. First, this was a retrospective observational study, and the individual physicians made all the treatment choices. Thus, there may have been a bias toward the choice of isatuximab treatment that could not be captured in the multivariate analysis. Second, because of the limited number of analyzed patients, we could not divide the patients into a derivation cohort and validation cohort for analysis. We adopted a bootstrap method for the internal validation, but it was difficult to confirm the external validation in our cohort. Therefore, we need to substantiate our results by other datasets. Third, we could not examine the effect of prior treatment on LMR and other laboratory data. Fourth, we could not analyze the detailed fractions of white blood cells, lymphocytes, and monocytes (such as CD4 + T cells, CD8 + T cells, regulatory T cell, natural killer cells, etc.) for a deeper understanding of the mechanism of isatuximab. Despite these limitations, we found that the patients with higher LMR (≥ 4) could obtain longer PFS and OS under the Isa-PD regimen. Other cohort studies of isatuximab treatment might be necessary to substantiate our results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was conducted with the support of members of the Kansai Myeloma Forum.
Abbreviations
- B2MG
β2 Microglobulin
- CI
Confidence interval
- CR
Complete response
- Isa-D
Isatuximab and dexamethasone
- Isa-KD
Isatuximab, carfilzomib and dexamethasone
- Isa-PD
Isatuximab, pomalidomide and dexamethasone
- ISS
International Staging system
- KMF
Kansai Myeloma Forum
- LMR
Lymphocyte/monocyte ratio
- MM
Multiple myeloma
- NA
Not available
- NLR
Neutrophile/lymphocyte ratio
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- SD
Stable disease
- VGPR
Very good partial response
- WBC
White blood cell
Author contributions
YS performed the research, collected data, analyzed data, and wrote the paper; JK performed the research, analyzed data, and critically reviewed and revised the paper. YO, S-iF, KO, YS, SK, RY, MM, HH, YA, NA, MH, KF, HY, SY, YT, TT, HT, HS, NU collected data and reviewed the paper. NH, TI, CS, IM, JK, AT-K and MH critically reviewed and revised the paper; All authors approved the submitted version.
Funding
KMF received research funding from Ono, Bristol-Myers Squibb, and AbbVie. This research was supported by Project Mirai Cancer Research Grants.
Data availability
The data of this study are available from the corresponding author, J. Kanda, upon reasonable request.
Declarations
Conflict of interest
Shin-ichi Fuchida has received honoraria from Takeda, Sanofi, Janssen, Ono, Bristol-Myers Squibb. Tomoki Ito has received honoraria from Bristol-Myers Squibb and Sanofi, Grant/Research funding from Bristol-Myers Squibb. Yuji Shimura has received honoraria from Bristol-Myers Squibb, Janssen Pharmaceutical and Sanofi. Teruhito Takakuwa has received honoraria from Bristol-Myers Squib and Grant/Research funding from Janssen Pharmaceutical and Sanofi. Hirohiko Shibayama reports honoraria from Takeda, Ono, Fujimoto, Janssen, Chugai, Eisai, Sanofi, AstraZeneca, Meiji Seika Pharma, and AbbVie. Chihiro Shimazaki has received honoraria from Janssen, Bristol-Myers Squibb, and Sanofi. Junya Kuroda is a consultant for Janssen Pharmaceutical and Bristol-Myers Squibb (BMS), and has received honoraria from Janssen Pharmaceutical, Ono Pharmaceutical, Sanofi, and BMS. The other authors have no conflict of interest.
Consent for publication
Not applicable.
Ethical approval and consent to participate
All procedures involving patients performed in this study were in accordance with the ethical standards of the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (approval no. R2887; approval date: January 6th, 2022) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The informed consent requirement for this retrospective study was waived because the study was conducted retrospectively and the opportunity to refuse was guaranteed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shimazu Y, Mizuno S, Fuchida S, et al. Improved survival of multiple myeloma patients treated with autologous transplantation in the modern era of new medicine. Cancer Sci. 2021;112:5034–5045. doi: 10.1111/cas.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/nejmoa1607751. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 4.Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397:2361–2371. doi: 10.1016/S0140-6736(21)00592-4. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos M, Bringhen S, Anttila P, et al. Isatuximab as monotherapy and combined with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2021;137:1154–1165. doi: 10.1182/blood.2020008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyos V, Borrello I. The immunotherapy era of myeloma: Monoclonal antibodies, vaccines, and adoptive T-cell therapies. Blood. 2016;128:1679–1687. doi: 10.1182/blood-2016-05-636357. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Otero P, Paiva B, Engelhardt M, et al. Is immunotherapy here to stay in multiple myeloma? Haematologica. 2017;102:423–432. doi: 10.3324/haematol.2016.152504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20:4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 9.Feng X, Zhang L, Acharya C, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res. 2017;23:4290–4300. doi: 10.1158/1078-0432.CCR-16-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin TG, Corzo K, Chiron M, et al. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cells. 2019;8:1522. doi: 10.3390/cells8121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30:399–408. doi: 10.1038/leu.2015.240. [DOI] [PubMed] [Google Scholar]
- 12.Shimazu Y, Kanda J, Kosugi S, et al. Efficacy of elotuzumab for multiple myeloma in reference to lymphocyte counts and kappa/lambda ratio or B2 microglobulin. Sci Rep. 2023;13:5159. doi: 10.1038/S41598-023-32426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimazu Y, Kanda J, Kaneko H, et al. Monocyte or white blood cell counts and β2 microglobulin predict the durable efficacy of daratumumab with lenalidomide. Ther Adv Hematol. 2022;13:20406207221142487. doi: 10.1177/20406207221142487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazu Y, Kitawaki T, Kondo T, Takaori-Kondo A. Pretreatment blast-to-lymphocyte ratio as a prognostic marker for CD19/CD3-bispecific T cell-engaging antibodies (blinatumomab) treatment against relapsed or refractory B-precursor acute lymphoblastic leukemia. Cancer Immunol Immunother. 2023;72:3861–3865. doi: 10.1007/s00262-023-03514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosani T, Covut F, Beck R, et al. Significance of the absolute lymphocyte/monocyte ratio as a prognostic immune biomarker in newly diagnosed multiple myeloma. Blood Cancer J. 2017;7:e579–e579. doi: 10.1038/bcj.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Nishiwaki K, Nagao R, et al. Clinical significance of the lymphocyte-to-monocyte ratio in multiple myeloma patients with negative minimal residual disease: a single-center retrospective analysis. Int J Hematol. 2021;114:599–607. doi: 10.1007/s12185-021-03201-y. [DOI] [PubMed] [Google Scholar]
- 17.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 18.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C-H, George SL. The bootstrap and identification of prognostic factors via cox’s proportional hazards regression model. Stat Med. 1985;4:39–46. doi: 10.1002/sim.4780040107. [DOI] [PubMed] [Google Scholar]
- 20.Efron B. Bootstrap methods: another look at the jackknife. New York: Springer; 1992. pp. 569–593. [Google Scholar]
- 21.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijhof IS, Casneuf T, Van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 23.Nijhof IS, Groen RWJ, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015;29:2039–2049. doi: 10.1038/leu.2015.123. [DOI] [PubMed] [Google Scholar]
- 24.Kararoudi MN, Nagai Y, Elmas E, et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood. 2020;136:2416–2427. doi: 10.1182/BLOOD.2020006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casneuf T, Adams HC, van de Donk NWCJ, et al. Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia. 2021;35:573–584. doi: 10.1038/s41375-020-0855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krejcik J, Frerichs KA, Nijhof IS, et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res. 2017;23:7498–7511. doi: 10.1158/1078-0432.CCR-17-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storti P, Vescovini R, Costa F, et al. CD14+CD16+ monocytes are involved in daratumumab-mediated myeloma cells killing and in anti-CD47 therapeutic strategy. Br J Haematol. 2020;190:430–436. doi: 10.1111/bjh.16548. [DOI] [PubMed] [Google Scholar]
- 28.Kumar SK, Dispenzieri A, Fraser R, et al. Early relapse after autologous hematopoietic cell transplantation remains a poor prognostic factor in multiple myeloma but outcomes have improved over time. Leukemia. 2018;32:523. doi: 10.1038/leu.2017.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available from the corresponding author, J. Kanda, upon reasonable request.