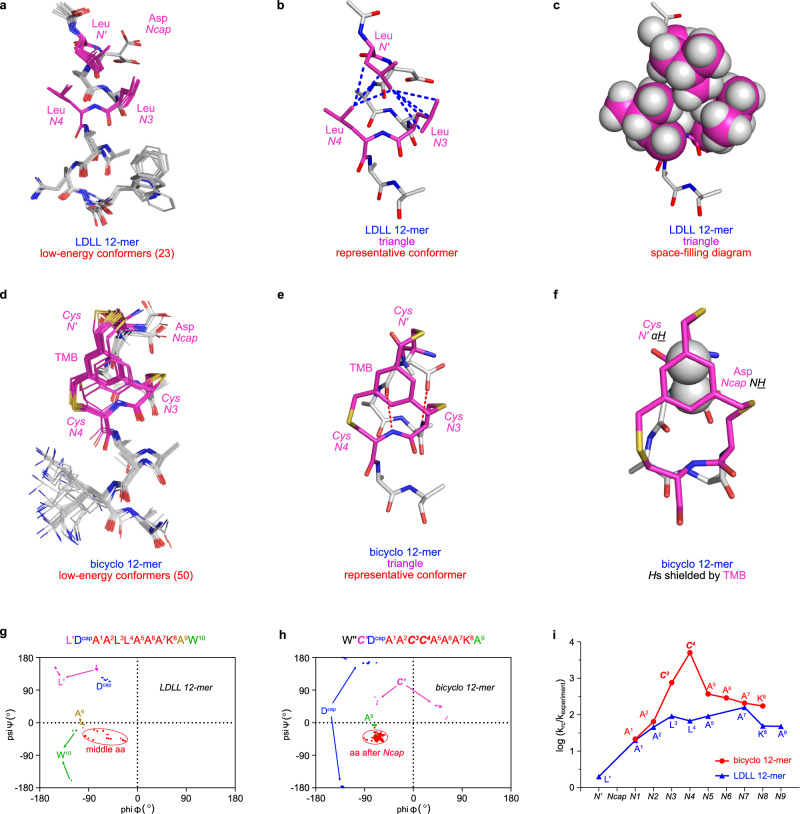
Fig. 5. Solution structures of LDLL and bicyclo 12-mers from conformational sampling with NMR constraints.
a Comprehensive ensemble of structures within 3 kcal•mol−1 of the minimum energy conformer located for the LDLL 12-mer. b, c Stick and space-filling diagrams of representative conformers of LDLL 12-mer highlighting three Leu side chains comprising the hydrophobic triangle. Blue dotted lines for hydrophobic interactions. d, e Corresponding diagrams for the bicyclo 12-mer where the TMB fragment mimics the hydrophobic triangle. Red dotted lines highlighting H-bonds in ASX motifs. f N’ Cys CαH and Ncap Asp NH of the bicyclo 12-mer are both shielded by TMB aromaticity, with Cα being closest. Ramachandran plots of the low energy conformers for linear LDLL (g), and bicyclo (h) 12-mer. i Protection factors for the bicyclo 12-mer around the capping region are significantly higher than for the linear LDLL 12-mer consistent with reduced H/D exchange rates corresponding to bicyclo N-cap rigidity. Source data are provided as a Source Data file.