Figure 7. Changes in CH50 versus platelet count over the course of the study in a phase 1 trial of sutimlimab in ITP.
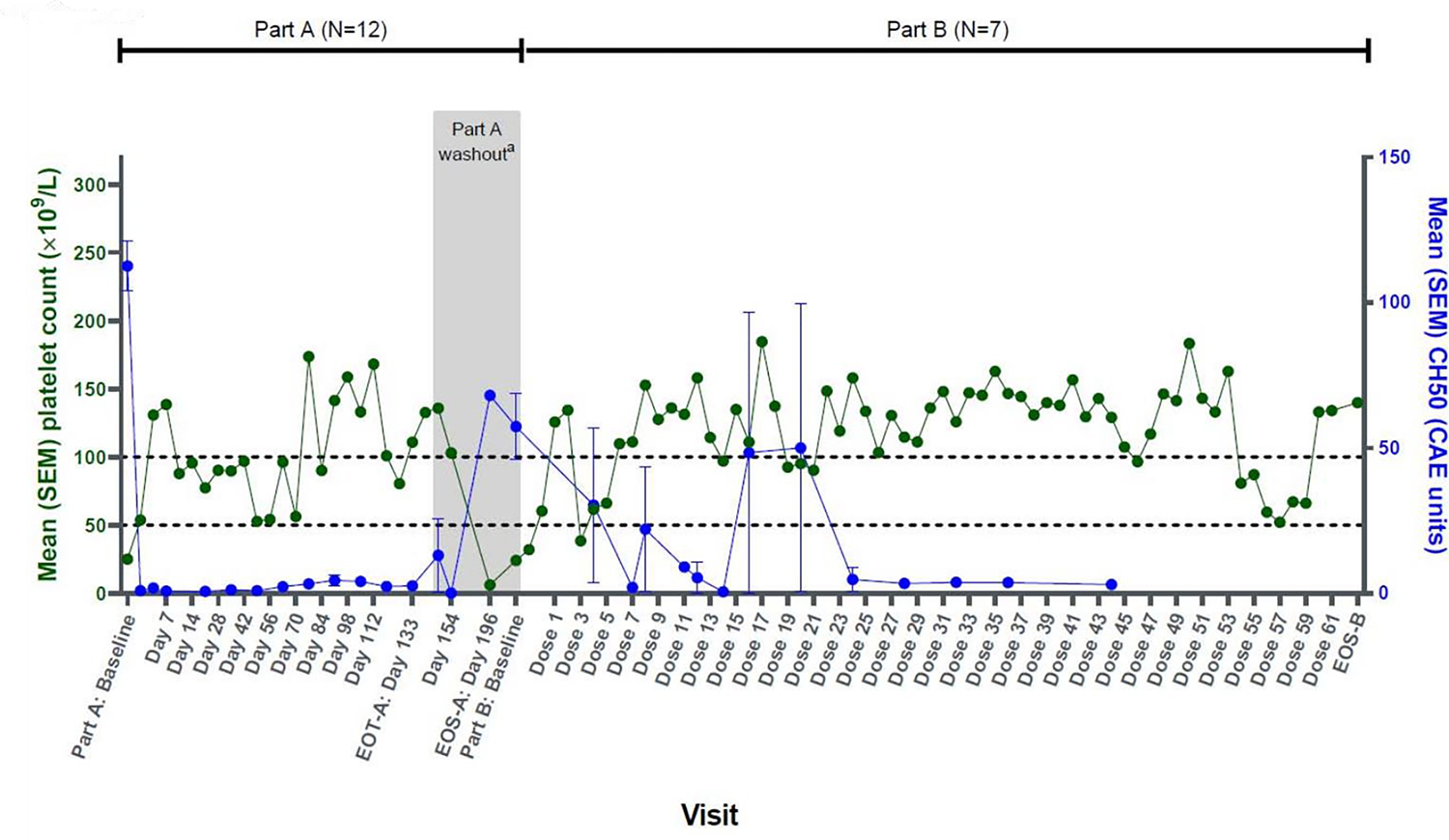
CAE, complement activity enzyme; EOS, end of study; EOT, end of treatment; SEM, standard error of the mean.
aFor patients enrolled in protocol version 3 or higher, washout period starts at Day 147 and ends at Day 196.
The value at Part A baseline is the average of all platelet counts during the screening period, including Day 0 predose. The value at Part B baseline is the average of all platelet counts during the screening period in Part B. Reproduced with permission from Broome et al (46).
