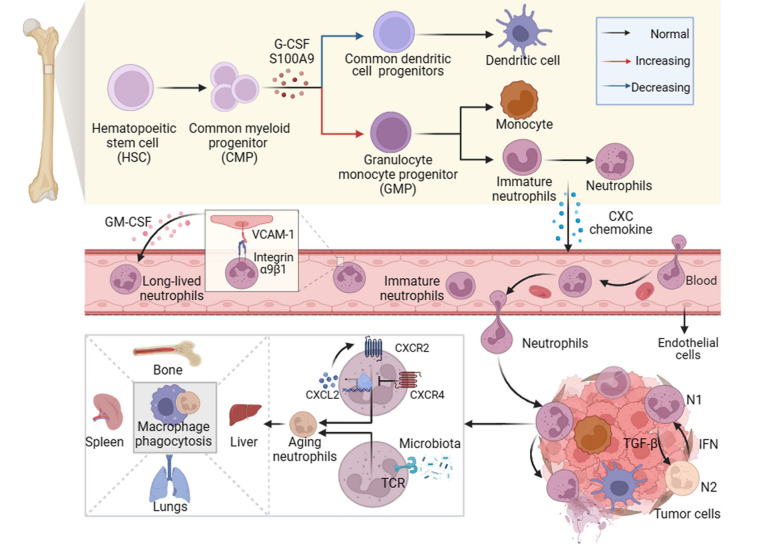
Fig. 1.
The development, mobilization, and clearance of neutrophils in the tumor microenvironment. Hematopoietic progenitor stem cells (HSCs) in the bone marrow differentiate into common myeloid progenitor cells (CMPs), which give rise to granulocyte-monocyte progenitor cells (GMPs) and eventually mature segmented neutrophils. Tumor-derived mediators such as granulocyte colony-stimulating factor (G-CSF) and S100 calcium-binding protein A9 (S100A9) promote the differentiation of the neutrophil and monocyte lineages while leading to systemic dendritic cell deficiency in vivo. Chemokines trigger the mobilization of mature and immature neutrophils into the circulation. Immature neutrophils, known as polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), are considered in this context. During transendothelial migration, the interaction between integrin α9β1 on neutrophils and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells stimulates the release of granulocyte-macrophage colony-stimulating factor (GM-CSF) from the latter, prolonging neutrophil lifespan. Neutrophils that extravasate into the tumor tissue adopt antitumor (type N1) or protumor (type N2) phenotypes, influenced by growth factor-β (TGF-β) and type 1 interferon (IFN), respectively. After fulfilling their functions, neutrophils undergo senescence due to intrinsic programs (CXCR2 or CXCR4) and extrinsic factors (microbiota) and are subsequently cleared by macrophages in the bone marrow, spleen, liver, and lungs. Neutrophils also undergo programmed death to form neutrophil extracellular traps (NETs)