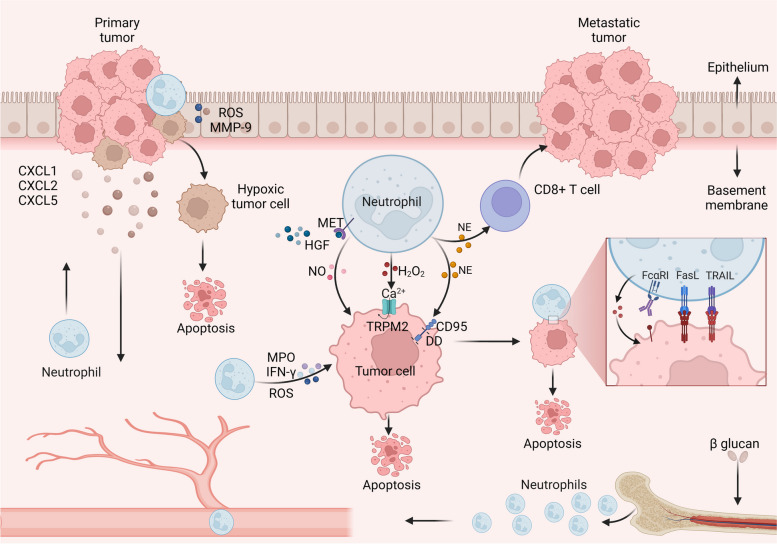
Fig. 2.
Direct cytotoxic effects of neutrophils on cancer cells. Neutrophils, when exposed to β-glucan, rely on the memory of bone marrow precursors and exert antitumor effects. The chemokines CXCL1, CXCL2, and CXCL5, secreted by primary tumors, facilitate the recruitment of neutrophils to the tumor site. Under hypoxic conditions, activated neutrophils induce reactive oxygen species (ROS) and matrix metalloproteinase (MMP-9) degradation of the epithelial basement membrane, which ultimately restricts tumor development. Neutrophils directly secrete ROS, myeloperoxidase (MPO), and interferon γ (IFN-γ) to inhibit tumor progression. The interaction between the ligand hepatocyte growth factor (HGF) and receptor tyrosine protein kinase (MET) on neutrophils leads to the release of nitric oxide (NO) by MET+ neutrophils, which exerts antitumor effects. Neutrophils secrete hydrogen peroxide (H2O2), inducing apoptosis in tumor cells through Ca2+ influx via the transient receptor potential cation channel, subfamily M, member 2 (TRPM2). Neutrophil elastase (NE) hydrolyzes and releases the CD95 death structure domain (DD), selectively killing cancer cells. Additionally, NE has distant effects on CD8+ T cells. Neutrophils with enhanced expression of TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) induce apoptosis in cancer cells through direct contact