FIGURE 4.
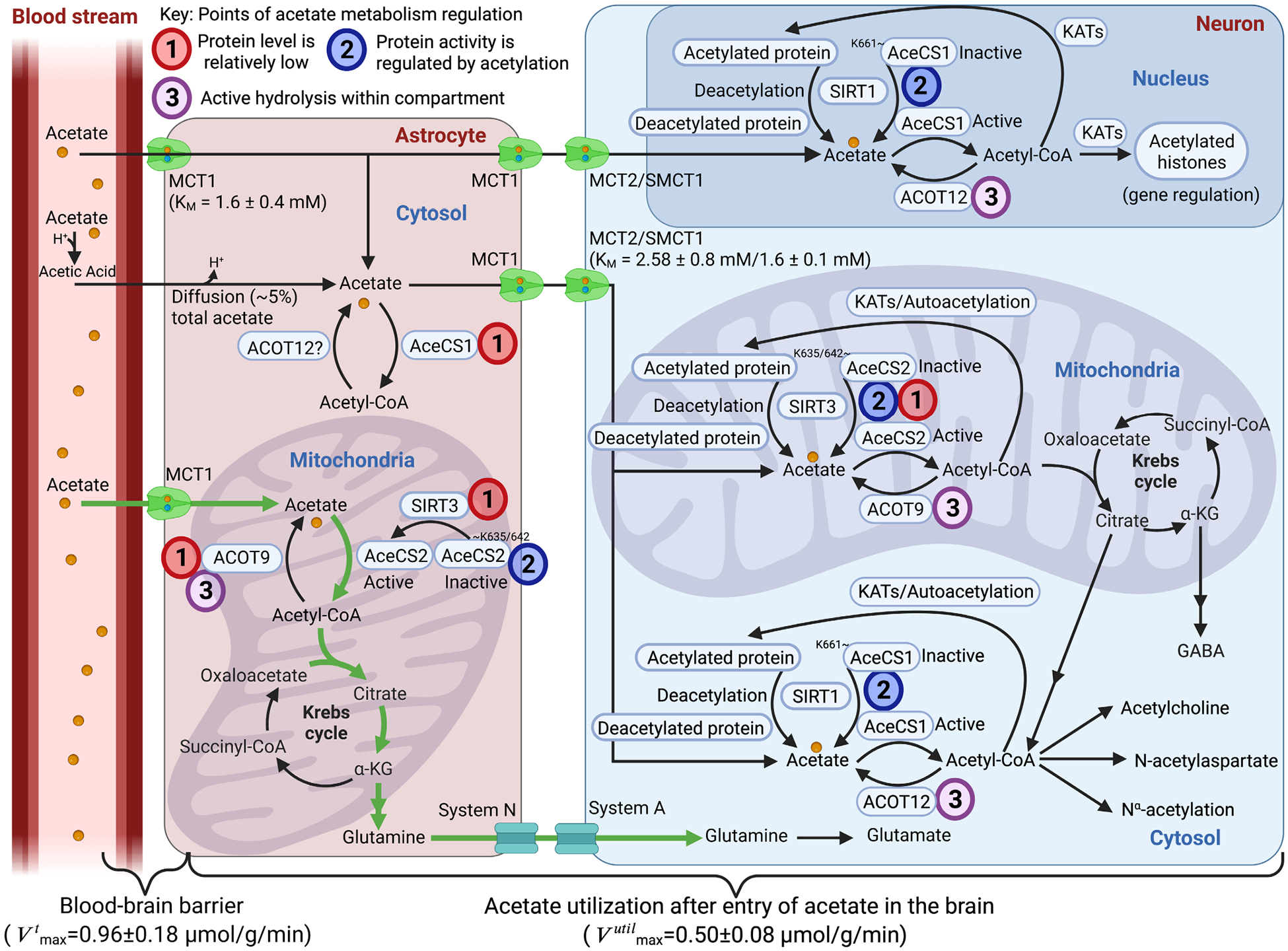
The transport of acetate into the brain exceeds the brain’s capacity to metabolize acetate. Acetate primarily enters the brain through monocarboxylate transporter 1 (MCT1) located in the astrocyte end feet in contact with blood vessels, a small portion of acetate can passively diffuse across the BBB in the form of acetic acid. Intracellular acetate is actively transported into the mitochondrial compartment and converted into acetyl-CoA by acetyl-CoA synthetase 2 (AceCS2; ACSS1, acyl-CoA synthetase short-chain family member 1). Entry of Acetyl-CoA into the astrocytic Krebs cycle can be used to synthesize glutamine which is transported into neurons for glutamate synthesis via the N and A transport systems (green arrows indicate primary use in brain). Regulation of this pathway is thought to be limited as both the protein levels of silent information regulator 3 (SIRT3), which regulates AceCS2 activity, and acyl-CoA thioesterase 9’s (ACOT9) hydrolytic activity is low compared to neurons. Acetate may also be exported through MCT1 located near the surface of neurons that can actively uptake acetate through MCT2/sodium MCT1. Neuronal acetate can be converted to acetyl-CoA in the cytosolic or nuclear compartments by AceCS1 (ACSS2, acyl-CoA synthetase short-chain family member 2), where it can be used by lysine acetyltransferases (KAT)s to acetylate histones for gene regulation, non-histone proteins, and metabolites and play a role in protein synthesis through Nα-acetylation. Acetyl-CoA may also be used for several biosynthetic pathways including the synthesis of acetylcholine and N-acetylaspartate for use in fatty acid synthesis. Acetate may also be actively transported into the neuronal mitochondrial compartment; however, the ability for neurons to use acetate for acetyl-CoA production may be limited in neurons due to the relatively low protein levels of AceCS2 and the relatively high levels of acetyl-CoA hydrolysis activity (ACOT9). However, as neurons are capable of using acetate under metabolically challenged conditions, neuronal capacity to convert acetate into Krebs cycle intermediates may be linked to the energy status of the cell, with neurons possessing relatively high SIRT3 protein levels, which would play a role in deacetylating and activating AceCS2 within neurons. Silent information regulator 1, SIRT1; acyl-CoA thioesterase 12, ACOT12; α-KG, α-Ketoglutarate; GABA, γ aminobutyric acid.
