Fig. 1. Centering and organizing the divisome.
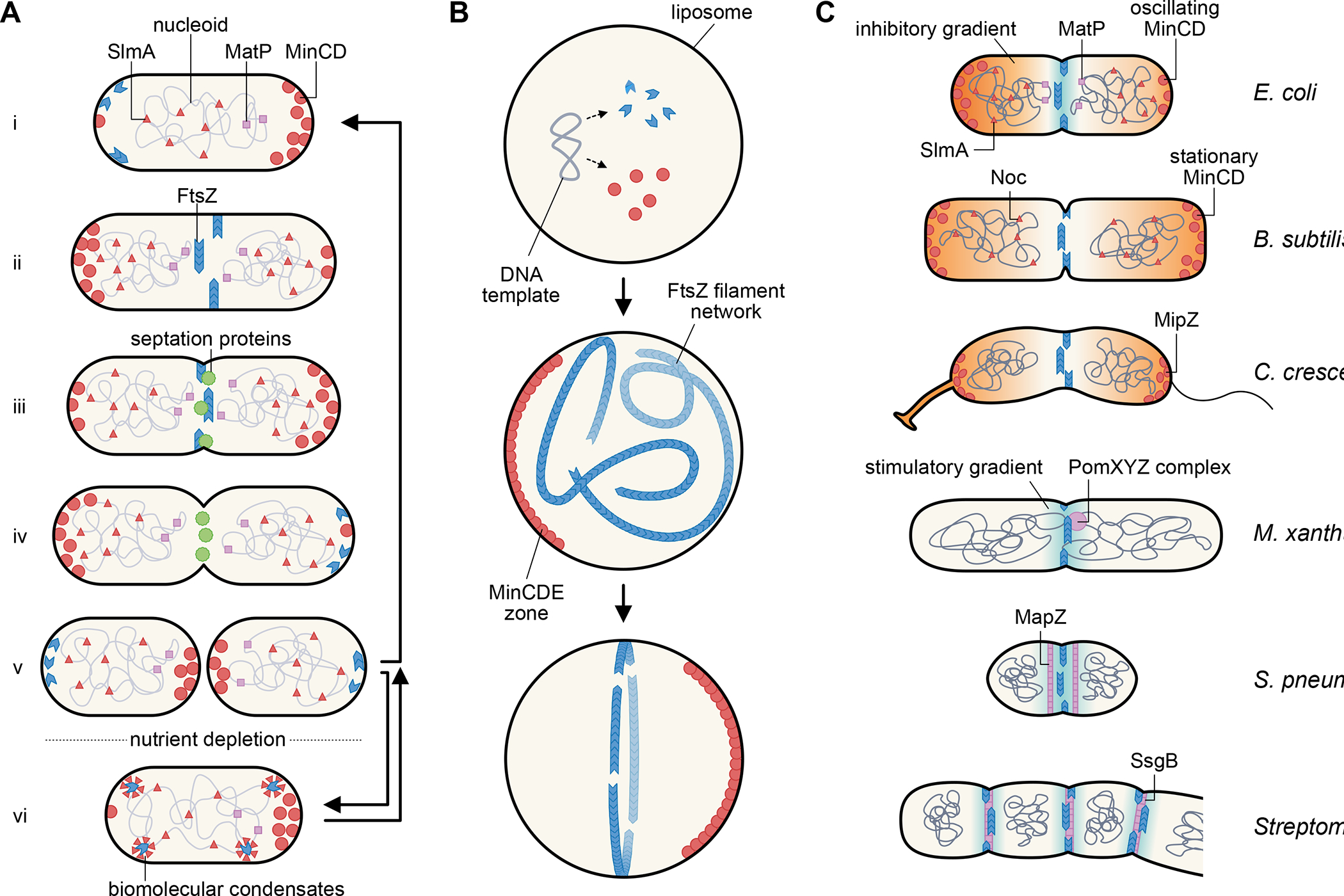
(A) Escherichia coli cell division cycle. Newborn cells (i) carry several spatial FtsZ positioning systems, including SlmA and MatP on the nucleoid and MinCD oscillating between the cell poles, that keep FtsZ from assembling into a Z ring at the cell poles or on top of the unsegregated nucleoid. As the replicated daughter nucleoids segregate, the Z ring assembles at midcell (ii) and recruits other septum-synthesis proteins (iii), which result in formation of the division septum. During septum synthesis, FtsZ leaves the division site prior to the other septation proteins (iv). The final step of cytokinesis results in two daughter cells (v), which begin the next cycle during growth conditions. Nutrient depletion or stress induces a quiescent state (vi) in which FtsZ localizes to putative biomolecular condensates that can revert back to polymer forms after growth restart. (B) Reconstitution of a centered proto-ring in lipid vesicles. After FtsZ, FtsA, and Min proteins are synthesized from a plasmid template or added as purified protein to lipid vesicles, they self-organize into membrane-associated polymers or gradients. Oscillation of MinCDE within the liposome helps to corral FtsA-tethered FtsZ polymers into a focused ring, mimicking in vivo behavior. (C) Theme and varations of Z ring positioning systems in diverse bacterial species, showing three examples of negative spatial regulators (orange gradients) and three examples of positive spatial regulators (blue gradients) and the proteins involved.
