Abstract
Several risk stratification systems aid clinicians in classifying pulmonary embolism (PE) severity and prognosis. We compared 2 clinical PE scoring systems, the PESI and sPESI scores, with 2 comorbidity indices, the Charlson Comorbidity Index (CCI) and the val Walraven Elixhauser Comorbidity Index (ECI), to determine the utility of each in predicting mortality and hospital readmission. Information was collected from 436 patients presenting with PE via retrospective chart review. The PESI, sPESI, CCI, and ECI scores were calculated for each patient. Multivariate analysis was used to determine each system's ability to predict in-hospital mortality, 90-day mortality, overall mortality, and all-cause hospital readmission. The impact of various demographic and clinical characteristics of each patient on these outcomes was also assessed. The PESI score was found to be an independent predictor of in-hospital mortality and 90-day mortality. The PESI score and the CCI were able to independently predict overall mortality. None of the 4 risk scores independently predicted hospital readmission. Other factors including hypoalbuminemia, serum BNP, coagulopathy, anemia, and diabetes were associated with increased mortality and readmission at various endpoints. The PESI score was the best tool for predicting mortality at any endpoint. The CCI may have utility in predicting long-term outcomes. Further work is needed to better determine the roles of the CCI and ECI in predicting patient outcomes in PE. The potential prognostic implications of low serum albumin and anemia at the time of PE also warrant further investigation.
Keywords: pulmonary embolism, Charlson Comorbidity Index, Elixhauser Comorbidity Index, risk stratification, mortality, hospital readmission
Introduction
Venous thromboembolism (VTE) is estimated to affect between 350,000 and 600,000 individuals annually in the United States. Pulmonary embolism (PE) is responsible for most of the mortality in cases of VTE. The US Surgeon General's office estimates the annual death rate in the United States attributable to PE to be within the range of 28,200 to 82,800. 1 Analysis of the National Hospital Ambulatory Medical Care Survey estimates that in the 5-year period from 2006 to 2010 there were 394,000 emergency department visits for PE of which 90% were admitted to the hospital. 2 It is widely accepted that PE is a common cause of mortality and potentially debilitating chronic conditions including post-pulmonary embolism syndrome and chronic thromboembolic pulmonary hypertension that may result in multiple hospitalizations.
Successful PE management involves the initial anticoagulant therapy in addition to various interventional or surgical approaches in selected patient groups. In the acute period mortality seems to be more related to the hemodynamic impact of the thromboembolic event. 3 Additionally, patients with PE often have comorbidities such as neoplasms, chronic cardiovascular or respiratory diseases, obesity, cirrhosis, and chronic kidney disease (CKD) which can predispose them to this condition and contribute to a worse short-term prognosis. The long-term prognosis of PE may also be influenced by these underlying comorbidities. Prognosis differs widely among PE patients and the impact of their comorbidity burden is not completely understood. The prediction of short-term and long-term risks is of paramount importance to help guide decision making in terms of intensity of the initial treatment, duration of therapy, and long-term follow-up in acute PE. There are not many studies on the impact of comorbidities in patients with PE as a tool to predict survival or hospital readmission. 4
Hospital readmissions are a preventable and expensive consequence after discharge. According to Medicare data approximately 15% of discharged patients are readmitted within the 30-day quality benchmark and 34% are readmitted within 90 days, resulting in a cost of 20 billion dollars annually. The disease factors most strongly associated with readmission in the general population are older age, multiple comorbidities, chronic disease, VTE, complications of cancer, and pneumonia. Despite this association hospital readmissions have not been well studied in acute PE patients.5-7
To better characterize the severity and prognosis of PE, several risk stratification systems have been developed. Standard classifications of PE severity include the American Heart Association/American College of Cardiology (AHA/ACC) and the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines.8,9 The most commonly used risk scores for predicting prognosis in PE are the Pulmonary Embolism Severity Index (PESI) and Simplified Pulmonary Embolism Severity Index (sPESI) scores. The PESI score uses 11 clinical and demographic pieces of information to stratify patients with acute PE into 5 classes that confer risk of 30-day mortality. 10 It has since been assessed for 90-day mortality, 6-month mortality, 1-year mortality, and hospital readmission.11,12 This index has been used to guide clinical decision-making regarding patients who may be suitable for early discharge and/or ambulatory treatment.13,14 The sPESI score, tailored to 6 variables, was introduced to provide clinicians with a more streamlined and efficient method of predicting short-term mortality risk in acute PE. This score was found to have similar prognostic accuracy to the PESI score in predicting 30-day mortality and has been assessed for other PE outcomes.12,15
The Charlson Comorbidity Index (CCI) is the most well-known score used to classify patients into risk categories according to medical comorbidities. The original CCI was developed for classifying prognostic comorbidities in longitudinal studies by using 17 common comorbid states. It has been validated to predict the risk of 10-year mortality in medical patients. 16 This index has been used for many clinical conditions and outcomes and has been examined within the context of acute PE. Another widely used method of quantifying comorbidity burden in hospitalized patients is the Elixhauser Comorbidity Index (ECI). Developed a decade after the CCI, this risk score identified 30 comorbidities that impact length of hospitalization, hospital resource use, and mortality. The ECI was originally intended to better assess how comorbid conditions contribute to a patient's use of healthcare resources and was not intended to be used as an index score. It was later modified by Van Walraven et al into a scoring system that, like the CCI, can summarize disease burden in an individual and be applied to outcomes such as mortality and hospital readmission.17,18 The ECI has not been evaluated for acute PE.
Patients presenting to the hospital with acute PE often have significant underlying medical comorbidities that both predispose them to PE and have a significant impact on their prognosis. The PESI and sPESI scores are commonly used in the acute setting to guide clinical decision making. There is a wealth of data surrounding the use of these scoring systems to predict short-term mortality in PE. Data regarding their applications to medium- and long-term mortality and hospital readmission in PE is limited. These scores also do not fully consider a patient's comorbidity burden. The aim of this study was to further investigate the comorbidities of acute PE patients and to evaluate the prognostic impact of the CCI and ECI, in comparison to the PESI and sPESI scores, to determine their utility in predicting outcomes related to mortality and all-cause hospital readmission in patients with acute PE.
Materials/Methods
Patient Selection and Data Collection
Data from 436 patients presenting with acute PE between May 2017 and August 2022 was collected following the ongoing institutional review board (IRB) and pulmonary embolism response team (PERT) protocols at Loyola University Medical Center and affiliated hospitals. Patients were considered to have a diagnosis of acute PE if a previously undiagnosed PE was radiographically confirmed by computed tomographic angiography or V/Q scintigraphy per admitting physician discretion.
Demographic and clinical information including age, sex, weight, and body mass index (BMI) was collected via retrospective chart review of electronic medical records (EMRs). Patients were stratified into subcategories according to the ACC/AHA (low risk, sub-massive, and massive) and ESC/ERS (low risk, intermediate-low risk, intermediate-high risk, and high risk) guidelines.8,9 Provoked VTE was classified as the occurrence of VTE in association with major transient or minor transient or persistent risk factors; VTE was classified as unprovoked in the absence of any identifiable risk factors. Risk factors for provoked PE including recent surgery within 2 months before PE, immobilization lasting longer than 4 days in the 2-month period before PE, the use of estrogen or oral contraceptive therapy before PE, and pregnancy or postpartum state before PE were collected from patient charts. A hypercoagulable state was defined as the presence of any risk factor at the time of PE (ie, active cancer, cirrhosis, nephrotic syndrome, antiphospholipid syndrome, acquired or hereditary thrombophilia, active COVID-19 infection) that causes exaggerated coagulation in the absence of bleeding. Active cancer was defined as newly diagnosed cancer (less than 3 months before), metastatic cancer or cancer with current therapy (ie, surgery, chemotherapy, radiotherapy, hormonal, immunotherapy, supportive therapy, or combined therapies). Although COVID-19 is not included in any of the risk scores that were evaluated, we noted all patients diagnosed with COVID-19 as the COVID-19 pandemic occurred during our study period. We considered COVID-19 to induce a hypercoagulable state but did not include it in calculation of any of the risk scores.
Vital signs related to hemodynamic function including heart rate, systolic blood pressure (SBP), respiratory rate (RR), oxygen saturation (SaO2), and body temperature were collected from patient charts at the time of PE diagnosis. Common clinical symptoms of PE were noted if present on admission including hemoptysis, dyspnea, syncope, chest pain, fever, and altered mental status (AMS). Data regarding the laboratory parameters of blood urea nitrogen (BUN), creatinine (Cr), d-dimer, troponin I, brain natriuretic peptide (BNP), lactate, and albumin was collected from the EMR at the time of PE diagnosis.
Risk Scores, Comorbidities, and Study Outcomes
A description of variables and assigned weighted points for all risk scores is available in the Supplemental appendix. A total point score for a given patient is obtained by summing the points for each applicable prognostic variable as assigned in each risk score. The PESI and sPESI scores were calculated as previously described.12,15 Each patient's CCI was calculated using the original weights assigned by Charlson et al using 17 relevant medical comorbidities. 16 The ECI was calculated using the weights assigned by Van Walraven et al using 30 relevant medical comorbidities. 18
The collected medical comorbidities were those needed to calculate the 4 risk scores and were comprised of smoking history, hypertension, coronary artery disease, congestive heart failure (CHF), cardiac arrhythmia, previous and new onset atrial fibrillation, peripheral artery disease, valvular heart disease, prior DVT, prior PE, prior VTE, chronic lung disease, chronic obstructive pulmonary disease (COPD), CKD, fluid electrolyte disorder, liver disease, peptic ulcer, cancer diagnosis, active/metastatic cancer, lymphoma diagnosis, diabetes mellitus, diabetes mellitus with microvascular complications (neuropathy, retinopathy, nephropathy), hypothyroidism, obesity, recent weight loss (≥ 10% of body weight), statin therapy, anemia/sickle cell disease, history of major bleeding, rheumatoid arthritis, cerebrovascular disease, cerebral ischemia, dementia/psychosis, depression, neurodegenerative disorders, organ transplantation, HIV/AIDS, alcohol abuse, and drug abuse. COVID-19 infection was also noted as described previously.
The primary study outcomes were the relationships between the 4 risk scores and mortality or all-cause hospital readmission after the index PE. Mortality was defined as in-hospital mortality, 90-day mortality, and overall mortality by using follow-up data from the EMRs. Hospital readmission was defined as any hospital admission following discharge from the index PE hospitalization. Secondary outcomes were the relationships between various demographic and clinical characteristics of these patients, including vital signs and serum biomarkers at time of PE diagnosis, and mortality or readmission.
Statistical Analysis
All calculations were performed with SPSS Statistics software (IBM). The Shapiro–Wilk and Kolmogorov–Smirnov tests were used to evaluate data distribution. Continuous variables in groups were non-normally distributed and were shown as median with interquartile ranges (IQR). Categorical variables were given as numbers with percentages. In univariate analysis differences in the study outcome events (in-hospital mortality, 90-day mortality, overall mortality, and hospital readmission) in different groups were evaluated by using non-parametric Mann–Whitney U-test for continuous variables and Chi-square and Fisher's exact tests (two-sided) for categorical variables. A receiver operating characteristic (ROC) curve analysis was performed for all of the tested risk scores to assess their predictive ability for the study outcomes. The optimal cutoff value of each risk score was assessed by calculating the corresponding area under the curve (AUC) and 95% confidence interval (CI).
Univariate Kaplan–Meier survival analysis with log-rank method was performed to assess unadjusted time-to-event outcomes from 2017 to the final study date of November 15, 2023 for overall mortality. Multivariate cox regression with a backward elimination method was performed to assess independent predictors of mortality outcomes. The independent predictors of hospital readmission were assessed by multivariate logistic regression analysis with a backward elimination method. Variables that were statistically significant with a 2-tailed P < .05 in univariate analysis were retained in the final multivariate models after multicollinearity assessment for each of the study outcomes. We used the lowest threshold value of 0.1 for tolerance values and the highest critical value of 5.0 for variance inflation factors (VIFs) for inclusion of variables to final multivariate models so that none of the variables exhibited multicollinearity at a concerning level. A P-value of <.05 was considered statistically significant.
Results
Clinical Characteristics of Acute PE Patients
The clinical characteristics of acute PE patients and their univariate analysis according to the study outcomes are presented in Table 1. The median follow-up period was 23 months (IQR: 17-49 months) after diagnosis. In the whole group the median age was 64.0 (IQR: 54-74) years. Two hundred fifteen patients were male (49.3%), and 221 patients were female (50.7%). Most of the acute PE patients were overweight or obese with a median BMI value of 29.73 (IQR: 24.93-35.68). Eighty-two (18.8%) patients had a history of recent surgery, 123 (18.2%) patients had a history of immobilization, 158 (36.2%) patients had an identifiable hypercoagulable state, and 249 (57.1%) patients had an identifiable etiology for provoked VTE. The frequencies of estrogen or oral contraceptive therapy use and pregnancy/postpartum state were low, observed in 8 (1.8%) and 3 (0.7%) patients respectively.
Table 1.
Clinical Characteristics of PE Patients and Univariate Analysis According to Clinical Outcomes.
| Variable | Whole Group (N = 436) | In-Hospital Mortality (N = 38) | 90-day Mortality (N = 57) | Overall Mortality (N = 102) | Readmission (N = 146) |
|---|---|---|---|---|---|
| Age (years), (median IQR) | 64.0 (54.0-74.0) | 66.0 (57.0-73.0) | 67.0 (59.0-73.0) | 68.0 (61.0-78.0)‡ | 64.5 (55.0-73.0) |
| Sex: Male, n (%) Female, n (%) | 215 (49.3%) 221 (50.7%) | 14 (36.8%)* 24 (63.2%)* | 21 (36.8%)* 36 (63.2%)* | 42 (41.2%) 60 (59.8%) | 60 (41.1%)* 86 (58.9%)* |
| Weight (kg) (median IQR) | 87.0 (70.35-105.97) | 73.95 (66.10-88.30)† | 70.80 (59.60-88.00)‡ | 73.15 (62.10-88.70)‡ | 87.25 (70.20-104.30) |
| BMI (kg/m2) (median IQR) | 29.73 (24.93-35.68) | 26.73 (21.46-30.97)* | 25.45 (21.41-32.28)‡ | 26.04 (22.53-31.47)‡ | 29.74 (25.21-35.67) |
| PE severity ACC/AHA | |||||
| Low risk, n (%) | 153 (35.1%) | 7 (18.4%)* | 12 (21.1%)* | 24 (23.5%)† | 45 (30.8%) |
| Submassive, n (%) | 248 (56.9%) | 24 (63.2%) | 37 (64.9%) | 66 (64.7%) | 92 (63.0%) |
| Massive, n (%) | 35 (8%) | 7 (18.4%)* | 8 (14.0%) | 12 (11.8%) | 9 (6.2%) |
| PE severity ESC | |||||
| Low risk, n (%) | 52 (11.9%) | 0 (0.0%) | 1 (1.8%) | 3 (2.9%) | 17 (11.6%) |
| Intermediate-Low, n (%) | 221 (50.7%) | 17 (44.7%) | 26 (45.6%) | 50 (49.0%) | 74 (50.7%) |
| Intermediate-High, n (%) | 128 (29.4%) | 14 (36.8%) | 22 (38.6%) | 37 (36.3%) | 46 (31.5%) |
| High risk, n (%) | 35 (8.0%) | 7 (18.4%)* | 8 (14.0%) | 12 (11.8%) | 9 (6.2%) |
| Additional risk factors | |||||
| Recent surgery, n (%) | 82 (18.8%) | 7 (18.4%) | 9 (15.8%) | 16 (15.7%) | 27 (18.5%) |
| Immobilization, n (%) | 123 (28.2%) | 13 (34.2%) | 18 (31.6%) | 32 (31.4%) | 34 (23.3%) |
| Estrogen therapy / Oral contraceptive, n (%) | 8 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pregnancy Postpartum, n (%) | 3 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) |
| Hypercoagulable state, n (%) | 158 (36.2%) | 25 (65.8%)‡ | 36 (63.2%)‡ | 55 (53.9%)‡ | 51 (34.9%) |
| Provoked VTE, n (%) | 249 (57.1%) | 30 (78.9%)† | 45 (78.9%)‡ | 79 (77.5%)‡ | 79 (54.1%) |
Comparisons between patients with versus without clinical outcome. *P < .05; †P < .01; ‡P < .001.
In-hospital mortality was observed in 38 (8.7%) patients, 90-day mortality was observed in 57 (13.1%) patients. A total of 102 (23.4%) patients were deceased during the entire study period. Female patients were more likely to experience mortality at any endpoint and this was statistically significant for both in-hospital mortality (36.8% male, 63.2% female, P < .05) and 90-day mortality (36.8% male, 63.2% female, P < .05). Body weight and BMI were lower in patients who experienced in-hospital mortality (body weight median: 73.95 kg, IQR: 66.10-88.30; BMI median: 26.73, IQR: 21.46-30.97), 90-day mortality (body weight median: 70.80 kg, IQR: 59.60-88.00; BMI median: 25.45, IQR: 21.41-32.28), and overall mortality (body weight median: 73.15 kg, IQR: 62.10-88.70; BMI median: 26.04, IQR: 22.53-31.47). The proportion of patients with a hypercoagulable state was higher in patients who experienced in-hospital mortality [25 patients (65.8%), P < .001], 90-day mortality [36 patients (63.2.%), P < .001], and overall mortality [55 patients (53.9%), P < .001]. The median age was significantly higher in the overall mortality group. Hospital readmission occurred in 146 (33.5%) acute PE patients, of which 86 (58.9%) patients were female and 60 (41.1%) patients were male (P < .05).
Vital Signs, Clinical Symptoms, and Laboratory Parameters of Acute PE Patients
Table 2 represents vital signs, clinical symptoms, and laboratory parameters of study patients and their univariate analysis according to the study outcomes. All the vital sign values were significantly associated with in-hospital mortality. However, this association was only significant for SBP and RR for 90-day mortality, SBP and body temperature for overall mortality, and heart rate for hospital readmission. The only clinical symptom with a statistically significant association with mortality at all 3 endpoints was presentation with AMS. No clinical symptoms correlated with hospital readmission. When examining laboratory parameters elevated BUN, troponin I, BNP, lactate, and low albumin were associated with an increased risk of mortality at all endpoints. Elevated BNP was associated with an increased risk of hospital readmission. There was no association between serum d-dimer or creatinine and any of these outcomes.
Table 2.
Vital Signs, Clinical Symptoms and Laboratory Parameters of PE Patients and Univariate Analysis According to Clinical Outcomes.
| Variable | Whole Group (N = 436) | In-Hospital Mortality (N = 38) | 90-day Mortality (N = 57) | Overall Mortality (N = 102) | Readmission (N = 146) |
|---|---|---|---|---|---|
| Vital signs | |||||
| Heart rate (BPM) (median IQR) | 95.00 (85.00-111.00) | 106.5 (93.00-118.00)† | 104.00 (89.00-114.00) | 100.50 (86.00-114.00) | 92.00 (82.00-105.00)* |
| Systolic blood pressure (mm Hg) (median IQR) | 116.50 (105.00-129.00) | 108.00 (94.00-125.00)† | 109.00 (96.00-120.00)† | 112.00 (99.00-128.00)* | 118.50 (106.00-131.00) |
| Respiratory rate (BMP) (median IQR) | 21.00 (18.00-25.00) | 24.00 (20.00-29.00)† | 22.00 (20.00-28.00)* | 22.00 (19.00-26.00) | 20.00 (18.00-24.00) |
| SaO2 (%) (median IQR) | 92.00 (90.00-94.00) | 91.50 (88.00-95.00)* | 92.00 (89.00-95.00) | 92.00 (88.00-94.00) | 92.00 (90.00-94.00) |
| Temperature (°C) (median IQR) | 36.8 (36.6-37.1) | 36.7 (36.4-36.9)* | 36.8 (36.6-36.9) | 36.7 (36.4-37.0)* | 36.8 (36.6-37.1) |
| Symptoms | |||||
| Hemoptysis, n (%) | 14 (3.2%) | 0 (0.0%) | 2 (3.5%) | 3 (2.9%) | 3 (2.1%) |
| Dyspnea, n (%) | 387 (88.8%) | 36 (94.7%) | 52 (91.2%) | 92 (90.2%) | 133 (91.1%) |
| Syncope, n (%) | 51 (11.17) | 8 (21.1%) | 9 (15.8%) | 14 (13.7%) | 13 (8.9%) |
| Chest pain, n (%) | 138 (31.7%) | 9 (23.7%) | 11 (19.3%)* | 18 (17.6%)‡ | 41 (28.1%) |
| Altered mental status, n (%) | 94 (21.6%) | 17 (44.7%)‡ | 22 (38.6%)‡ | 31 (30.4%)† | 24 (16.4%) |
| Lower limb DVT, n (%) | 216 (49.5%) | 14 (36.8%) | 24 (42.1%) | 46 (45.1%) | 76 (52.1%) |
| Laboratory parameters | |||||
| BUN (mg/dl) (median IQR) | 16.00 (11.00-23.00) | 25.00 (15.00-33.00)† | 24.00 (15.00-32.00)‡ | 22.00 (15.00-32.00)‡ | 16.00 (12.50-23.00) |
| Creatinine (mg/dl) (median IQR) | 0.98 (0.75-1.20) | 1.10 (0.82-1.63) | 1.04 (0.80-1.60) | 1.07 (0.86-1.48) | 0.99 (0.79-1.24) |
| D-dimer (ng/ml) (median IQR) | 5132.50 (1855.25-14516.00) | 9973.00 (2310.50-46313.50) | 5418.00 (2178.00-19959.00) | 4304.00 (1946.00-16312.00) | 5308.00 (2048.50-13875.50) |
| Troponin I (ng/ml) (median IQR) | 0.04 (0.03-0.17) | 0.11 (0.05 −0.72)‡ | 0.09 (0.04-0.22)‡ | 0.05 (0.03-0.14)* | 0.05 (0.03-0.17) |
| BNP (pg/ml) (median IQR) | 115.00 (45.75-380.25) | 448.00 (81.50-1105.00)‡ | 411.00 (106.00-1118.00)‡ | 279.00 (90.50-1051.00)‡ | 175.00 (67.00-357.00)† |
| Lactate (mmol/L) (median IQR) | 1.40 (1.10-2.10) | 2.50 (1.60-4.70)‡ | 2.00 (1.50-3.20)‡ | 2.00 (1.45-3.25)‡ | 1.50 (1.20-2.10) |
| Albumin (g/dl) (median IQR) | 3.30 (2.80-3.70) | 3.00 (2.50-3.40)‡ | 3.00 (2.50-3.40)‡ | 3.00 (2.50-3.40)‡ | 3.30 (2.90-3.50) |
Comparisons between patients with versus without clinical outcome. *P < .05; †P < .01; ‡P < .001.
Comorbidities of Acute PE Patients
Table 3 shows the comorbidities of acute PE patients and their univariate analysis according to the study outcomes. History of cancer, active cancer, obesity, recent weight loss, and anemic state including sickle cell disease were significantly associated with all mortality endpoints. Fluid electrolyte disorders were significantly associated with in-hospital and 90-day mortality. Congestive heart failure, cardiac arrhythmia, CKD, history of major bleeding, and organ transplantation were significantly associated with overall mortality. Hypertension, congestive heart failure, CKD, liver disease, diabetes and its complications, statin therapy, anemic state including sickle cell disease, and history of organ transplantation were significantly associated with hospital readmission.
Table 3.
Comorbidities of PE Patients and Univariate Analysis According to Clinical Outcomes.
| Parameter | Whole Group (N = 436) | In-Hospital Mortality (N = 38) | 90-day Mortality (N = 57) | Overall Mortality (N = 102) | Readmission (N = 146) |
|---|---|---|---|---|---|
| Active smoker, n (%) | 67 (15.4%) | 6 (15.8%) | 9 (15.8%) | 15 (14.7%) | 26 (17.8%) |
| Hypertension, n (%) | 240 (55.0%) | 20 (52.6%) | 30 (52.6%) | 61 (59.8%) | 91 (62.3%)* |
| Coronary artery disease, n (%) | 53 (12.2%) | 3 (7.9%) | 6 (10.5%) | 14 (13.7%) | 21 (14.4%) |
| Congestive heart failure, n (%) | 66 (15.1%) | 5 (13.2%) | 13 (22.8%) | 26(25.5%)† | 33 (22.6%)† |
| Cardiac arrhythmia, n (%) | 55 (12.6%) | 8 (21.1%) | 11 (19.3%) | 20 (19.6%)* | 20 (13.7%) |
| History of atrial fibrillation, n (%) | 47 (10.8%) | 7 (18.4%) | 9 (15.8%) | 15 (14.7%) | 17 (11.6%) |
| New onset atrial fibrillation, n (%) | 14 (3.2%) | 2 (5.3%) | 3 (5.3%) | 6 (5.9%) | 5 (3.4%) |
| Peripheral artery disease, n (%) | 9 (2.1%) | 1 (2.6%) | 1 (1.8%) | 2 (2.0%) | 4 (2.7%) |
| Valvular heart disease, n (%) | 22 (5.0%) | 1 (2.6%) | 4 (7.0%) | 9 (8.8%) | 11 (7.5%) |
| Prior DVT, n (%) | 64 (14.7%) | 3 (7.9%) | 5 (8.8%) | 11 (10.8%) | 24 (16.4%) |
| Prior PE, n (%) | 40 (9.2%) | 2 (5.3%) | 3 (5.3%) | 7 (6.9%) | 14 (9.6%) |
| Prior VTE, n (%) | 70 (16.1%) | 4 (10.5%) | 7 (12.3%) | 13 (12.7%) | 28 (19.2%) |
| Chronic lung disease, n (%) | 78 (17.9%) | 6 (15.8%) | 12 (21.1%) | 24 (23.5%) | 33 (22.6%) |
| COPD, n (%) | 33 (7.6%) | 2 (5.3%) | 5 (8.8%) | 11 (10.8%) | 16 (11.0%) |
| Chronic kidney disease, n (%) | 41 (9.4%) | 6 (15.8%) | 8 (14.0%) | 17 (16.7%)† | 20 (13.7%)* |
| Fluid electrolyte disorder, n (%) | 66 (15.1%) | 12 (31.6%)† | 16 (28.1%)† | 19 (18.6%) | 27 (18.5%) |
| History of liver disease, n (%) | 25 (5.7%) | 2 (5.3%) | 2 (3.5%) | 8 (7.8%) | 13 (8.9%)* |
| History of peptic ulcer, n (%) | 10 (2.3%) | 0 (0.0%) | 2 (3.5%) | 2 (2.0%) | 3 (2.1%) |
| History of cancer, n (%) | 137 (31.4%) | 21 (55.3%)† | 33 (57.9%)‡ | 62 (60.8%)‡ | 46 (31.5%) |
| Active/Metastatic cancer, n (%) | 91 (20.9%) | 20 (52.6%)‡ | 30 (52.6%)‡ | 52 (51.0%)‡ | 34 (23.3%) |
| Lymphoma, n (%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | 1 (0.7%) |
| Diabetes, n (%) | 98 (22.5%) | 10 (26.3%) | 13 (22.8%) | 23 (22.5%) | 46 (31.5%)† |
| Diabetes with complications, n (%) | 26 (6.0%) | 4 (10.5%) | 5 (8.8%) | 8 (7.8%) | 14 (9.6%)* |
| Hypothyroidism, n (%) | 44 (10.1%) | 1 (2.6%) | 2 (3.5%) | 8 (7.8%) | 16 (11.0%) |
| Obesity, n (%) | 214 (49.1%) | 12 (31.6%)* | 19 (33.3%)* | 34 (33.3%)‡ | 72 (49.3%) |
| Recent weight loss, n (%) | 52 (11.9%) | 9 (23.7%)* | 16 (28.1%)‡ | 22 (21.6%)‡ | 20 (13.7%) |
| History of statin therapy, n (%) | 143 (32.8%) | 10 (26.3%) | 13 (22.8%) | 34 (33.3%) | 61 (41.8%)† |
| History of anemia / sickle cell, n (%) | 56 (12.8%) | 9 (23.7%)* | 16 (28.1%)‡ | 25 (24.5%)‡ | 26 (17.8%)* |
| History of major bleeding, n (%) | 39 (8.9%) | 5 (13.2%) | 8 (14.0%) | 16 (15.7%)† | 18 (12.3%) |
| Rheumatoid arthritis, n (%) | 6 (1.4%) | 0 (0.0%) | 1 (1.8%) | 2 (2.0%) | 3 (2.1%) |
| Cerebrovascular disease, n (%) | 67 (15.4%) | 9 (23.7%) | 13 (22.8%) | 21 (20.6%) | 23 (15.8%) |
| Cerebral ischemia, n (%) | 53 (12.2%) | 8 (21.1%) | 11 (19.3%) | 17 (16.7%) | 18 (12.3%) |
| History of dementia/Psychosis, n (%) | 26 (6.0%) | 2 (5.3%) | 4 (7.0%) | 6 (5.9%) | 9 (6.2%) |
| History of depression, n (%) | 35 (8.0%) | 0 (0.0%) | 3 (5.3%) | 8 (7.8%) | 15 (10.3%) |
| History of neurodegenerative disorder, n (%) | 16 (3.7%) | 1 (2.6%) | 2 (3.5%) | 2 (2.0%) | 4 (2.7%) |
| History of organ transplantation, n (%) | 9 (2.1%) | 1 (2.6%) | 1 (1.8%) | 5 (4.9%)* | 6 (4.1%)* |
| History of HIV/AIDS, n (%) | 5 (1.1%) | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | 1 (0.7%) |
| History of alcohol abuse, n (%) | 62 (14.2%) | 6 (15.8%) | 9 (15.8%) | 15 (14.7%) | 17 (11.6%) |
| History of drug abuse, n (%) | 41 (9.4%) | 1 (2.6%) | 3 (5.3%) | 5 (4.9%) | 14 (9.6%) |
| COVID-19 infection, n (%) | 38 (8.7%) | 3 (7.9%) | 3 (5.3%) | 4 (3.9%) | 9 (6.2%) |
Comparisons between patients with versus without clinical outcome. *P < .05; †P < .01; ‡P < .001.
Risk Scores of Acute PE Patients
Table 4 shows the PESI, sPESI, CCI, and ECI risk scores of study patients and their univariate analysis for each endpoint. In the univariate analysis, the PESI, sPESI, and ECI risk scores were all significantly higher in patients who experienced in-hospital, 90-day, and overall mortality but not in patients with hospital readmission. The CCI was significantly higher in patients who met any of the 3-mortality endpoints as well as who experienced hospital readmission.
Table 4.
Risk Scores of Acute PE Patients and Univariate Analysis According to Clinical Outcomes.
| Variable | Whole Group (N = 437) | In-Hospital Mortality (N = 38) | 90-day Mortality (N = 57) | Overall Mortality (N = 102) | Readmission (N = 146) |
|---|---|---|---|---|---|
| Pulmonary Embolism Severity Index (PESI) (median, IQR) | 105.00 (77.00-141.00) | 150.00 (118.00-179.00)‡ | 141.00 (117.00-175.00)‡ | 141.00 (111.00-173.00)‡ | 103.00 (78.00-136.00) |
| Simplified PESI Score (sPESI) (median, IQR) | 1.50 (1.00-2.00) | 2.50 (2.00-3.00)‡ | 2.00 (2.00-3.00)‡ | 2.00 (2.00-3.00)‡ | 1.50 (1.00-2.00) |
| Charlson Score (median, IQR) | 3.00 (1.00-6.00) | 6.00 (2.00-8.00)‡ | 6.00 (2.00-8.00)‡ | 6.00 (2.00-8.00)‡ | 3.00 (1.00-6.00)* |
| Elixhauser Score (median, IQR) | 12.00 (4.00-19.00) | 19.00 (14.00-25.00)‡ | 20.00 (15.00-25.00)‡ | 19.00 (15.00-26.00)‡ | 14.00 (5.00-19.00) |
Comparisons between patients with versus without clinical outcome. *P < .05; †P < .01; ‡P < .001.
ROC Curve Analysis
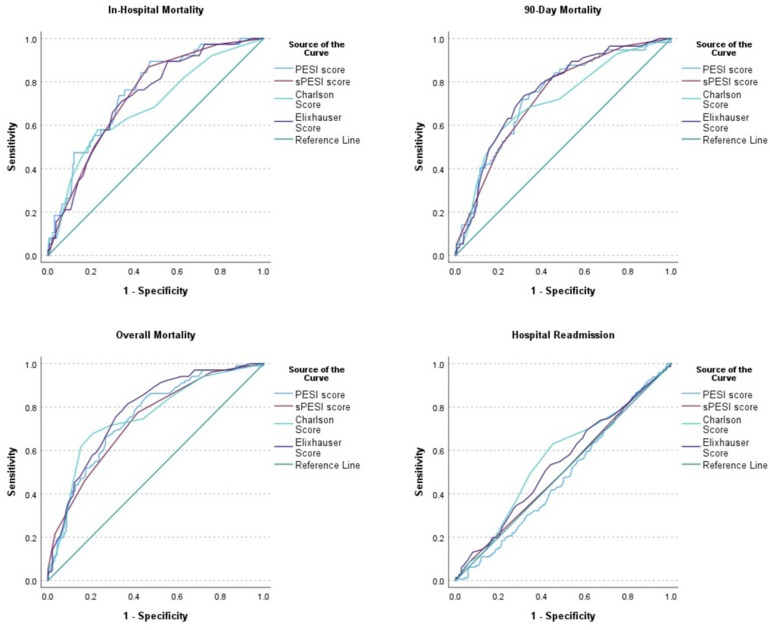
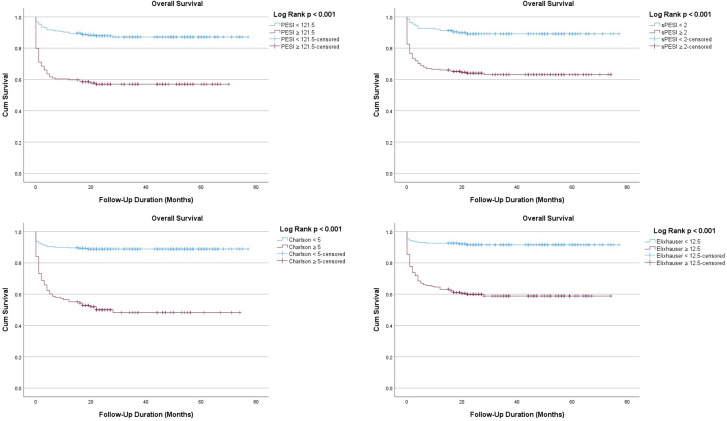
Table 5 and Figure 1 show the ROC curve analysis of the risk scores for each of the study outcomes. The ROC curve analysis was significant for all mortality outcomes with close AUC values. The AUC value of the PESI was highest for in-hospital mortality (AUC: 0.754, 95% CI: 0.680-0.827, P < .001). The AUC value of ECI was highest for 90-day (AUC: 0.751, 95% CI: 0.688-0.814, P < .001) and overall (AUC: 0.778, 95% CI: 0.730-0.826, P < .001) mortality. Although the predictive strength was poor, the AUC value of CCI was highest (AUC: 0.564, 95% CI: 0.506-0.621, P = .03) for hospital readmission while the other risk scores were not significant for this outcome. Figure 2 shows the Kaplan–Meier survival curves using the best cut-off values of the risk scores for overall mortality.
Table 5.
ROC Curve Analysis of Risk Scores and Comorbidity Indices for Clinical Outcomes.
| Parameter | In-Hospital Mortality (N = 38) | 90-day Mortality (N = 57) | Overall Mortality (N = 102) | Readmission (N = 146) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI; P-Value) | Best Cut-off | Sensitivity- Specificity | AUC (95% CI; P-Value) | Best Cut-off | Sensitivity- Specificity | AUC (95% CI; P-Value) | Best Cut-off | Sensitivity- Specificity | AUC (95% CI; P-Value) | Best Cut-off | Sensitivity- Specificity | |
| Pulmonary Embolism Severity Index (PESI) | 0.754 (0.680, 0.827, P < .001) | 104.50 | 0.895-0.528 | 0.735 (0.668, 0.802 P < .001) | 121.50 | 0.719-0.689 | 0.751 (0.700, 0.803, P < .001) | 121.50 | 0.663-0.728 | 0.481 (0.424-0.537, P = .49) | 68.5 | 0.849-0.179 |
| Simplified PESI Score (sPESI) | 0.735 (0.662, 0.808, P < .001) | 2.0 | 0.868-0.535 | 0.728 (0.663, 0.793, P < .001) | 2.0 | 0.825-0.549 | 0.736 (0.682, 0.791, P < .001) | 2.0 | 0.772-0.584 | 0.509 (0.451-0.566, P = .76) | 4.0 | 0.089-0.931 |
| Charlson Score | 0.686 (0.593-0.778, P < .001) | 6.0 | 0.579-0.709 | 0.709 (0.635-0.783, P < .001) | 6.0 | 0.579-0.786 | 0.755 (0.700-0.810, P < .001) | 5.0 | 0.676-0.793 | 0.564 (0.506-0.621, P = .03) | 3.0 | 0.630-0.548 |
| Elixhauser Score | 0.723 (0.647, 0.799, 0.001) | 15.50 | 0.711-0.658 | 0.751 (0.688, 0.814, P < .001) | 15.50 | 0.737-0.681 | 0.778 (0.730, 0.826, P < .001) | 12.50 | 0.814-0.632 | 0.541 (0.483-0.598, P = .16) | 12.5 | 0.534-0.559 |
Figure 1.
Receiver operating characteristic curves of the risk scores.
Figure 2.
Kaplan–Meier curves using the best cut-off values of the risk scores for overall mortality.
Multivariate Analysis
Table 6 shows the multivariate analysis of the study outcomes. Among the risk scores, only the PESI score (≥ 104.50) (HR 3.98; 95% CI 1.15-13.73; P = .03) remained an independent predictor of in-hospital mortality. The PESI score (≥ 121.50) (HR 2.12; 95% CI 1.12-4.03; P = .02) was also an independent predictor of 90-day mortality. Both PESI score (≥ 121.50) (HR 2.37; 95% CI 1.47-3.82; P < .001) and CCI (≥ 5.0) (HR 3.13; 95% CI 1.99-4.91; P < .001) were independent predictors for overall mortality. None of the risk scores independently predicted hospital readmission.
Table 6.
Results of Multivariable Analysis.
| A—Multivariable analysis for in-hospital mortality | |||||
|---|---|---|---|---|---|
| Coefficient (B) | Standard Error of B | Odds Ratio | 95% Confidence Interval | P-Value | |
| Albumin | −0.925 | 0.282 | 0.40 | 0.23-0.69 | < .001 |
| SaO2 (%) | −0.051 | 0.023 | 0.95 | 0.90-0.99 | .02 |
| BNP | 0.0002 | 0.00006 | 1.0002 | 1.0001-1.0003 | < .001 |
| Hypercoagulable state | 1.120 | 0.383 | 3.07 | 1.45-6.49 | < .01 |
| PESI score ≥ 104.5 | 1.380 | 0.632 | 3.98 | 1.15-13.73 | .03 |
| Variables included in the model: Sex, weight, ACC/AHA low risk, ACC/AHA massive, hypercoagulable state, provoked VTE, HR, SBP, RR, SaO2, Temperature, AMS, BUN, troponin I, BNP, lactate, albumin. Fluid electrolyte disorder, cancer diagnosis, obesity, recent weight loss, history of anemia, PESI ≥ 104.5, sPESI ≥ 2, CCI ≥ 6, ECI ≥15.5. | |||||
| B—Multivariable analysis for 90-day mortality | |||||
| Albumin | −0.800 | 0.230 | 0.450 | 0.29-0.71 | < .001 |
| History of anemia/Sickle | 0.641 | 0.317 | 1.90 | 1.02-3.53 | .04 |
| BNP | 0.00015 | 0.00005 | 1.0002 | 1.000041-1.00026 | < .001 |
| Hypercoagulable state | 0.901 | 0.302 | 2.46 | 1.36-4.44 | < .01 |
| PESI Score ≥ 121.5 | 0.752 | 0.328 | 2.12 | 1.12-4.03 | .02 |
| Variables included in the model: Sex, weight, ACC/AHA low risk, hypercoagulable state, provoked VTE, SBP, RR, chest pain, AMS, BUN, troponin I, BNP, lactate, albumin. Fluid electrolyte disorder, cancer diagnosis, obesity, recent weight loss, history of anemia, PESI ≥121.5, sPESI ≥ 2, CCI ≥6, ECI ≥15.5. | |||||
| C—Multivariable analysis for overall mortality | |||||
| Albumin | −0.564 | 0.177 | 0.57 | 0.40-0.80 | < .01 |
| Weight | −0.021 | 0.006 | 0.98 | 0.97-0.99 | < .01 |
| BUN | 0.014 | 0.006 | 1.014 | 1.003-1.03 | .01 |
| BNP | 0.00011 | 0.00006 | 1.0001 | 1.000005-1.00022 | .02 |
| History of anemia/Sickle | 0.536 | 0.250 | 1.71 | 1.05-2.79 | .03 |
| Cardiac arrhythmia | 0.604 | 0.272 | 1.83 | 1.07-3.11 | .03 |
| PESI Score ≥ 121.5 | 0.865 | 0.243 | 2.37 | 1.47-3.82 | < .001 |
| Charlson Score ≥ 5 | 1.142 | 0.230 | 3.13 | 1.99-4.91 | < .001 |
| Variables included in the model: Age, weight, ACC/AHA low risk, hypercoagulable state, provoked VTE, SBP, temperature, chest pain, AMS, BUN, troponin I, BNP, lactate, albumin, CHF, Cardiac arrythmia, CKD, cancer diagnosis, obesity, recent weight loss, history of anemia, history of major bleeding, organ transplantation, PESI ≥ 121.5, sPESI ≥ 2, CCI ≥ 5, ECI ≥ 12.5. | |||||
| D—Multivariable analysis for hospital readmission | |||||
| Male sex | −0.443 | 0.219 | 0.64 | 0.42-0.99 | .04 |
| Heart rate | −0.014 | 0.006 | 0.99 | 0.97-0.99 | .02 |
| History of DM | 0.616 | 0.256 | 1.85 | 1.12-3.05 | .02 |
| Variables included in the model: Sex, Heart rate, BNP, Hypertension, CHF, CKD, liver disease, diabetes, statin therapy, anemia, organ transplantation, CCI ≥ 3. | |||||
Additionally, serum albumin (HR 0.40; 95% CI 0.23-0.69; P < .001), BNP (HR 1.0002; 95% CI 1.0001-1.0003; P < .001), oxygen saturation at admission (HR 0.95; 95% CI 0.90-0.99; P < .02), and the presence of a hypercoagulable state (HR 3.07; 95% CI 1.45-6.49; P < .01) were significant predictors of in-hospital mortality. Serum albumin (HR 0.450; 95% CI 0.29-0.71; P < .001), BNP (HR 1.0002; 95% CI 1.000041-1.00026; P < .001), the presence of a hypercoagulable state (HR 2.46; 95% CI 1.36-4.44; P < .01), and the presence of an anemic state (HR 1.90; 95% CI 1.02-3.53; P < .04) were associated with 90-day mortality. Serum albumin, BNP, the presence of anemic state, recent weight loss (HR 0.98; 95% CI 0.97-0.99; P < .01), elevated serum BUN (HR 1.014; 95% CI 1.003-1.03; P = .01), and cardiac arrhythmia (HR 1.83; 95% CI 1.07-3.11; P = .03) were significant predictors of overall mortality.
Male patients (adjusted OR = 0.64; 95% CI = 0.42-0.99; P = .04) had lower odds of being readmitted to the hospital, as did those with increased heart rate (HR 0.99; 95% CI 0.97-0.99; P = .02). Only the presence of diabetes mellitus increased the odds of hospital readmission (OR = 1.85; 95% CI = 1.12-3.05; P = .02).
Discussion
Accurate risk stratification is critical to the management of patients with acute PE. A variety of risk scores and classification systems exist to aid clinicians in assessing potential outcomes in patients presenting with PE. The PESI and sPESI scores were designed and validated for risk of 30-day mortality in PE patients.10,15 The creators of the CCI validated their system using the risk of death 1 year after hospital admission and then assessed its ability to predict 10-year mortality. 16 Van Walraven's modification of the Elixhauser Comorbidity Measure was also originally validated for in-hospital mortality. 18 To our knowledge no studies have attempted to determine the utility of the ECI in assessing outcomes for acute PE. The majority of existing studies that have directly compared the predictive power of the Charlson and Elixhauser indices have focused on their application to postoperative surgical complications and not a specific medical disease state.19,20 The prognostic ability of these 2 comorbidity indices has never been formally compared to that of the PESI/sPESI scores in PE patients. By comparing each of these risk scores in a real-world patient population, we hope to provide clinicians with additional data and tools that may be used when caring for a patient with acute PE.
Prior studies have assessed the CCI in predicting outcomes in PE. Acute PE can be closely related to or provoked by a variety of medical comorbidities with which a patient may present. Ng et al examined a large cohort of Australian patients over a 7-year period and found that patients with a CCI ≥1 were more likely to die in the hospital or after discharge when compared to those patients with a CCI score of 0. 21 These findings were confirmed by Golpe et al in the same patient cohort. 22 In a similar study examining 176 patients who presented to a Chinese hospital between 2010 and 2012, patients with a CCI ≥1 were more likely to have died 2 years after hospital discharge and each 1-point increase in the CCI score carried a 1.76 increased risk of mortality at this endpoint. 23 A recently published study by Keller et al analyzing a nationwide cohort of German patients also found higher comorbidity burden according to the CCI to be related to the risk of in-hospital mortality. 24 These findings are concordant with those in our univariate analysis, in which a CCI ≥ 6.00 was associated with in-hospital, 90-day, and overall mortality. In the multivariate analysis only the PESI score demonstrated an independent association with in-hospital or 90-day mortality and the CCI was also able to independently predict overall mortality.
To our knowledge, only 1 study has previously sought to compare a clinical PE scoring system, sPESI, with a comorbidity index, the CCI. Friz et al compared the abilities of the sPESI and the CCI to predict medium- or long-term mortality in elderly patients with acute PE at 3 months, 6 months, 1, 2, and 5 years. They found that a CCI ≥ 1.00 was able to independently predict mortality at each of these endpoints and that sPESI failed to predict mortality at each of these endpoints. 25 We also did not find the sPESI score able to independently predict mortality at any endpoint in our multivariate analysis, however in our cohort the PESI score was independently associated with mortality at both short-, medium-, and long-term endpoints. The CCI was independently associated with overall mortality. The study by Friz did not include the original PESI score.
The better prognostic ability of the PESI score than the sPESI score was particularly interesting to us. Our findings differ from those published by Dentali et al who determined that the PESI and sPESI scores have similar prognostic ability at 3 months but not at 1 year. 12 Several factors may account for this. Each component of the sPESI score is weighted equally. The PESI score assigns different weights to each component and notably AMS carries the highest weight. In our cohort, AMS was the only clinical symptom of PE significantly associated with all 3 mortality endpoints in the univariate analysis. The PESI score also assigns a high weight to a history of malignancy which was significantly associated with the 3 mortality endpoints in our univariate analysis.
Another significant finding was the inability of the ECI to independently predict any of the outcomes we assessed. In the univariate analysis, the ECI had the highest discriminatory power for predicting 90-day mortality (AUC 0.751; 95% CI 0.688-0.814, P < .001) and overall mortality (AUC 0.778, 95% CI 0.730-0.826, P < .001). Why this did not translate to significance in the multivariate analysis warrants further study but may be due to the differential weights, including negative weights, assigned by val Walraven to each comorbidity.
We assessed the ability of each scoring system to predict all-cause hospital readmission given the high rates of readmission experienced by PE patients. A recent analysis of the National Readmission Database from 2010 and 2018 found the readmission rate for PE to range from 9.7% to 11.8%. 26 There are limited studies that have assessed the ability of the PESI, sPESI, and CCI to predict readmission. To our knowledge, none have applied the ECI to this outcome.11,27-29 In our study only the CCI was associated with readmission in the univariate analysis with poor discriminatory power. The ECI was not associated with readmission. This may indicate that patients with a higher burden of medical comorbidities are more likely to eventually be readmitted to the hospital, but it was surprising that the ECI did not reinforce this finding. The only medical comorbidity that was able to independently predict readmission in the multivariate analysis was a history of diabetes mellitus. Diabetes is well established as both a risk factor for recurrent PE and as a driver of a variety of vascular diseases that may prompt hospital readmission in these patients.30,31
Another important component of our study was the assessment of best cut-off points for each scoring system. Unlike the PESI and sPESI scores, the CCI and ECI do not have set cut-off points that can be used to quickly assess the risk of patient outcomes in the acute setting of PE. In the ROC curve analysis, the best cut-off point for mortality at each endpoint for the CCI ranged from 5.0 to 6.0 indicating the presence of multiple medical comorbidities or a highly weighted comorbidity such as metastatic cancer. This held true for the best cut-off points for mortality at each endpoint for the ECI, which ranged from 12.5 to 15.5. For hospital readmission the best cut-off point for the CCI was 3.0, suggesting a lower comorbidity burden may be associated with need for re-hospitalization at any point when compared to the comorbidity burden that increases risk of mortality.
There are other exploratory findings to discuss from our study. In the multivariate analysis, anemia (excluding blood loss and including sickle cell disease) demonstrated independent predictive ability for 90-day and overall mortality. Evidence on the prognostic value of anemia in PE remains limited but aligns with our findings. Several studies have found associations between anemia in PE patients and an increased rate of 30-day mortality and longer length of hospitalization.32,33 Hemodynamic changes associated with anemia may be better tolerated by a healthy heart than by a heart experiencing right ventricular strain due to PE. 33 The presence of a hypercoagulable state also demonstrated an independent association with both in-hospital mortality and 90-day mortality. Large studies examining the prevalence of the Elixhauser comorbidities in VTE patients have found coagulopathy to be an independent predictor of short-term mortality. 34 The role of anemia and hypercoagulability in determining outcomes in PE patients warrants further investigation.
In the multivariate analysis, a decrease in serum albumin or elevation in serum BNP was associated with mortality at all endpoints. The relationship between elevated BNP and right ventricular strain in PE and its use in predicting adverse outcomes is well established.35,36 Albumin is a major determinant of plasma oncotic pressure and blood viscosity. It has significant antioxidant and anti-inflammatory effects as well as a regulatory role within the vascular endothelium.37,38 Despite these ties to pathophysiologic mechanisms of thrombosis serum albumin is not frequently used as a prognostic measure in the management of acute PE. Hypoalbuminemia has been associated with higher risk of massive PE. 39 Recent studies have sought to better establish a connection between levels of serum albumin at time of PE and patient outcomes. Hoskin et al found hypoalbuminemia in patients admitted with acute PE to be independently associated with both 30-day and 90-day mortality which was reinforced in our study. 37 Hypoalbuminemia has also been associated with long-term mortality in PE which was reinforced by our findings. 40 Serum albumin may have underutilized value in predicting outcomes in PE patients and this warrants additional investigation.
We believe that our findings affirm those of Friz and colleagues. In the acute setting, altered hemodynamics and changes in cardiopulmonary-associated biomarkers may better predict PE outcomes but within the context of long-term mortality underlying medical comorbidities are more important drivers of patient outcomes. Our findings build on the current literature by including the ECI and by directly comparing these 4 risk stratification systems in PE patients for the first time. We found the PESI score to be the most consistent tool for assessing mortality at any endpoint and the CCI to be the best tool for assessing risk of overall mortality. Other independent predictors of mortality in our study include elevated BNP, hypoalbuminemia, coagulopathy, and anemia. It seems possible that new scoring indices for acute PE that incorporate these biomarker derangements and relevant comorbidities could be studied with the goal of continuing to refine our ability to risk stratify these patients in both the acute and long-term settings. None of the tools examined demonstrated predictive ability for hospital readmission but the role of chronic comorbid conditions such as diabetes should be further explored for this outcome.
Our study has limitations which should be noted. This is a retrospective study conducted at a single center. The small sample size limits the generalizability of our results. This may also contribute to variance between the CIs obtained in our study and those found in similar literature on this topic. Larger multi-center trials are needed to better assess the potential clinical applications of our findings. In our study, we assessed overall mortality and hospital readmission as events that occurred at any point after the index PE in order to best capture if patients met these outcomes. Further refinement of this data is needed to assess which patients may have died or been readmitted at specific times, such as 1 year or 5 years. Our data was limited to a retrospective review of our EMR, and it is possible that review of an epidemiological follow-up service (ie, the Social Security Death Index or the National Death Index) may have elucidated additional data including deaths outside of our center. Reviewing the ICD 9/10 codes for medical comorbidities for each patient could help to refine our data in the future as well. We did not analyze cause-specific mortality or readmission as our primary focus was on overall comorbidity burden as quantified by the CCI and ECI. This may be an important possible future application of our results. We chose to analyze hospital readmission as a binary outcome (any readmission or no readmissions) as some patients in our cohort had none, one, or multiple readmissions after the index PE. We did not consider time-event associations for readmission in this study, but this may be another possible future application of our data. The 4 risk scores were calculated at the time of PE diagnosis, and it is possible that future events may have resulted in patients acquiring new comorbidities over time that we were unable to account for in our long-term outcomes. In our multivariate analysis, there was concern for multicollinearity. However, tolerance values ranged from 0.309 to 0.970, all above the commonly referenced threshold of 0.1, indicating that none of the variables exhibit multicollinearity at a concerning level. The VIF values ranged from 1.031 to 3.231, which did not exceed the commonly used critical value of 5. This suggests that, while some multicollinearity may exist, it is not severe, potentially having limited impact on the models. This also suggests that the models are robust.
Our study also has several strengths. All of the patients assessed presented to and were evaluated at the same medical center within a 4-year window. Workup for each patient followed the standardized Loyola University Medical Center PERT team protocols. Standardized documentation by the PERT team ensured that demographic and clinical information from each chart was collected in a similar and comprehensive manner. The PERT team closely follows these patients both in the hospital and at regular outpatient follow-up appointments. The PERT program is still ongoing and recruiting additional patients and this data may be used to better contextualize the findings of our study in the future.
Summary and Conclusion
Risk stratification in acute PE is a continuously evolving process. We sought to compare 2 clinical PE scoring systems, the PESI and sPESI, with 2 comorbidity indices, the CCI and ECI, to see if any were able to predict in-hospital, 90-day, and overall mortality as well as all-cause hospital readmission in a real-world cohort of PE patients. The PESI score was able to independently predict in-hospital, 90-day, and overall mortality. The CCI was also able to independently predict overall mortality. The sPESI and ECI were not able to independently predict mortality at any endpoint. None of these systems were able to independently predict hospital readmission. Elevated BNP, hypoalbuminemia, coagulopathy, and anemia also had an independent association with patient mortality. Further work is needed to better determine the roles of the CCI and ECI in predicting patient outcomes in PE. The prognostic implications of low serum albumin and anemia at time of PE warrant investigation moving forward.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296241253844 for Charlson and Elixhauser Comorbidity Indices for Prediction of Mortality and Hospital Readmission in Patients With Acute Pulmonary Embolism by Alexander O’Hara, Jacob Pozin, Mohammed Abourahma, Ryan Gigstad, Danny Torres, Benji Knapp, Bulent Kantarcioglu, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors are thankful to the staff of the Hemostasis and Thrombosis Research laboratories for their assistance in completing the reported studies. We are also thankful to the Cardiovascular Institute for its support and partially funding these studies. The skillful assistance of Ms. Erin Healy Erickson in preparing this manuscript is greatly appreciated.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexander O’Hara https://orcid.org/0009-0007-1856-4292
Bulent Kantarcioglu https://orcid.org/0000-0003-3060-721X
Jawed Fareed https://orcid.org/0000-0003-3465-2499
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Office of the Surgeon General (US); 2008. [PubMed] [Google Scholar]
- 2.Singer AJ, Thode HC, Jr, Peacock WF. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: A US perspective. Clin Exp Emerg Med. 2016;3(3):126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit K, 'Arsigny D, L C. Risk stratification of acute pulmonary embolism. J Thromb Haemost. 2023;21(11):3016-3023. [DOI] [PubMed] [Google Scholar]
- 4.Dentali F, Cei M, Mumoli N, Gianni M. How to predict short- and long-term mortality in patients with pulmonary embolism? Pol Arch Med Wewn. 2015;125(1-2):82-88. [DOI] [PubMed] [Google Scholar]
- 5.Welker C, Huang J, Elmadhoun O, Esmaeilzadeh S, Mookadam F, Ramakrishna H. Morbidity following pulmonary embolism hospitalization - contributing factors and outcomes. J Cardiothorac Vasc Anesth. 2024;38(5):1239–1243. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients [published correction appears in JAMA Intern Med. 2016 Oct 1;176(10):1579]. JAMA Intern Med. 2016;176(4):484-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011 Apr 21;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428. [DOI] [PubMed] [Google Scholar]
- 8.Russell C, Keshavamurthy S, Saha S. Classification and stratification of pulmonary embolisms. Int J Angiol. 2022 Sep 2;31(3):162-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association [published correction appears in Circulation. 2012 Aug 14;126(7):E104] [published correction appears in Circulation. 2012 Mar 20;125(11):E495]. Circulation. 2011;123(16):1788-1830. [DOI] [PubMed] [Google Scholar]
- 10.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041-1046. doi:10.1164/rccm.200506-862OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golamari RR, Roy SG, Zheng S, Alvarez M, Ramireddy K, Pedersen C. Can pulmonary embolism severity index (PESI) score predict readmission rates in patients with pulmonary embolism? [abstract]. Circulation. 2019;140(Suppl_1):A15537. [Google Scholar]
- 12.Dentali F, Riva N, Turato S, et al. Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J Thromb Haemost. 2013;11(12):2103-2110. [DOI] [PubMed] [Google Scholar]
- 13.Smith V, Salinas-Abedalaziz R, Shannon M, Madden B. The use of pulmonary embolism severity index (PESI) score in identifying patients suitable for ambulatory treatment or early hospital discharge following diagnosis of pulmonary embolism. Eur Respir J. 2012;40(Suppl 56):p411. [Google Scholar]
- 14.Jiménez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132(1):24-30. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383-1389. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. [DOI] [PubMed] [Google Scholar]
- 19.Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maron SZ, Neifert SN, Ranson WA, et al. Elixhauser comorbidity measure is superior to Charlson comorbidity index in predicting hospital complications following elective posterior cervical decompression and fusion. World Neurosurg. 2020;138:e26-e34. [DOI] [PubMed] [Google Scholar]
- 21.Ng AC, Chow V, Yong AS, Chung T, Kritharides L. Prognostic impact of the charlson comorbidity index on mortality following acute pulmonary embolism. Respiration. 2013;85(5):408-416. [DOI] [PubMed] [Google Scholar]
- 22.Golpe R, Pérez-de-Llano LA, Castro-Añón O. Prognostic value of the Charlson comorbidity index in pulmonary embolism. Respiration. 2013;85(5):438-438. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Tang Y, Wang L, Shi C, Feng Y, Yi Q. Risk factors associated with long-term mortality in patients with pulmonary embolism and the predictive value of Charlson comorbidity index. Zhonghua Yi Xue Za Zhi. 2016;96(4):273-276. [DOI] [PubMed] [Google Scholar]
- 24.Keller K, Schmitt V, Espinola-Klein C, et al. Comorbidity burden assessed by the Charlson comorbidity index and its influence on prognosis of patients with pulmonary embolism. Eur Heart J. November 2023;44(Supplement 2). [Google Scholar]
- 25.Polo Friz H, Orenti A, Gelfi E, et al. Predictors of medium- and long-term mortality in elderly patients with acute pulmonary embolism. Heliyon. 2020;6(9):e04857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murthi M, Velagapudi S, Park DY, Shaka H. Pulmonary embolism readmission trend over the years (from a National Readmission Database). Am J Cardiol. 2022;184:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Kohn CG, Weeda ER, Kumar N, et al. External validation of a claims-based and clinical approach for predicting post-pulmonary embolism outcomes among United States veterans. Intern Emerg Med. 2017;12(5):613-619. [DOI] [PubMed] [Google Scholar]
- 28.Ho TA, Lio KU, Shah A, et al. Charlson comorbidity index predicts readmission risk following acute pulmonary embolism hospitalization. Chest. 2023;164(4):a5924. [Google Scholar]
- 29.Prajapati D, Suryanarayan D, Lang ES. P118: Pulmonary embolism severity index (PESI) score as a predictor for readmission in acute pulmonary embolism in emergency department? CJEM. 2018;20(S1):S98-S99. [Google Scholar]
- 30.Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48(5):1017-1021. [DOI] [PubMed] [Google Scholar]
- 31.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. South tees diabetes mortality study. Cause-specific mortality in a population with diabetes: South tees diabetes mortality study. Diabetes Care. 2002;25(1):43-48. [DOI] [PubMed] [Google Scholar]
- 32.Jiménez D, Escobar C, Martí D, et al. Association of anaemia and mortality in patients with acute pulmonary embolism. Thromb Haemost. 2009;102(1):153-158. [DOI] [PubMed] [Google Scholar]
- 33.Donzé J, Labarère J, Méan M, Jiménez D, Aujesky D. Prognostic importance of anaemia in patients with acute pulmonary embolism. Thromb Haemost. 2011;106(2):289-295. [DOI] [PubMed] [Google Scholar]
- 34.Tsai J, Grant AM, Soucie JM, et al. Clustering patterns of comorbidities associated with in-hospital death in hospitalizations of US adults with venous thromboembolism. Int J Med Sci. 2013;10(10):1352-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coutance G, Le Page O, Lo T, Hamon M. Prognostic value of brain natriuretic peptide in acute pulmonary embolism. Crit Care. 2008;12(4):R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: A systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178(4):425-430. [DOI] [PubMed] [Google Scholar]
- 37.Hoskin S, Chow V, Kritharides L, Ng ACC. Incidence and impact of hypoalbuminaemia on outcomes following acute pulmonary embolism. Heart Lung Circ. 2020;29(2):280-287. [DOI] [PubMed] [Google Scholar]
- 38.Shannon CM, Ballew SH, Daya N, et al. Serum albumin and risks of hospitalization and death: Findings from the atherosclerosis risk in communities study. J Am Geriatr Soc. 2021;69(10):2865-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omar HR, Mirsaeidi M, Rashad R, et al. Association of serum albumin and severity of pulmonary embolism. Medicina (Kaunas). 2020;56(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanık VO, Çınar T, Karabağ Y, et al. The prognostic value of the serum albumin level for long-term prognosis in patients with acute pulmonary embolism. Clin Respir J. 2020;14(6):578-585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296241253844 for Charlson and Elixhauser Comorbidity Indices for Prediction of Mortality and Hospital Readmission in Patients With Acute Pulmonary Embolism by Alexander O’Hara, Jacob Pozin, Mohammed Abourahma, Ryan Gigstad, Danny Torres, Benji Knapp, Bulent Kantarcioglu, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis