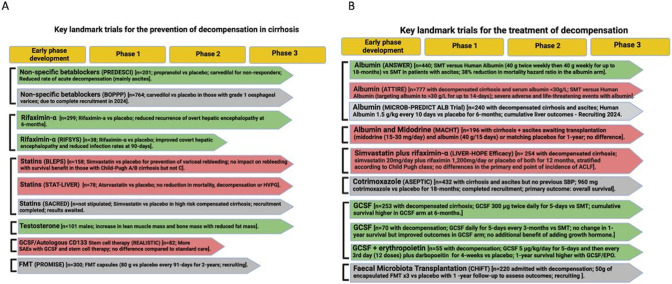
Figure 3.
Key landmark clinical trials in cirrhosis in patients with compensated cirrhosis and clinically significant portal hypertension (A) and in patients with decompensated cirrhosis (B). (A) Illustrates recently published phase 2a/b and 3 clinical trials in patients with compensated cirrhosis and clinically significant portal hypertension with the outcome of improved decompensation-free survival. These include trials of non-specific beta blockers, rifaximin-α, statins, testosterone, granulocyte colony-stimulating factor (GCSF)/autologous CD133 stem cell therapy and faecal microbiota transplantation (FMT). The trials highlighted in green bars are positive, light red bars negative and in grey bars are still recruiting or await reporting. (B) Illustrates recently published phase 2a/b and 3 clinical trials in patients with decompensated cirrhosis with the main outcome of survival. These include trials of human albumin solution, midodrine, simvastatin, cotrimoxazole, GCSF and GCSF in combination with growth hormone/erythropoietin. HVPG, hepatic venous pressure gradient; SAEs, serious adverse events; SBP, spontaneous bacterial peritonitis; SMT, standard medical therapy. Created with BioRender.com with publication licence.