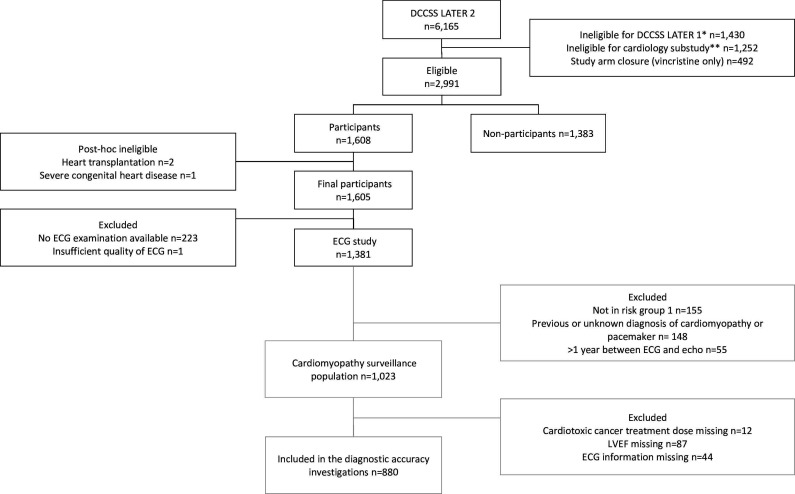
Figure 1.
Flow chart of the study. *Examples of ineligibility criteria include: refusal of study participation, deceased, lost to follow-up and living abroad. **Survivors who did not fall into one of the following risk groups: risk group 1 survivors who received anthracyclines, mitoxantrone or chest-directed radiotherapy; risk group 2 (max n=100): cyclophosphamide only (no anthracyclines, mitoxantrone, or chest-directed radiotherapy, ifosfamide or vincristine); risk group 3 (max n=100): ifosfamide only (no anthracyclines, mitoxantrone, or chest-directed radiotherapy, cyclophosphamide or vincristine); risk group 4 (max n=100): vincristine only (no anthracyclines, mitoxantrone, or chest-directed radiotherapy, ifosfamide or cyclophosphamide).25 DCCSS LATER 2, Dutch Childhood Cancer Survivor Study, LATER cohort (1963–2001) part 2; LVEF, left ventricular ejection fraction; n, number.