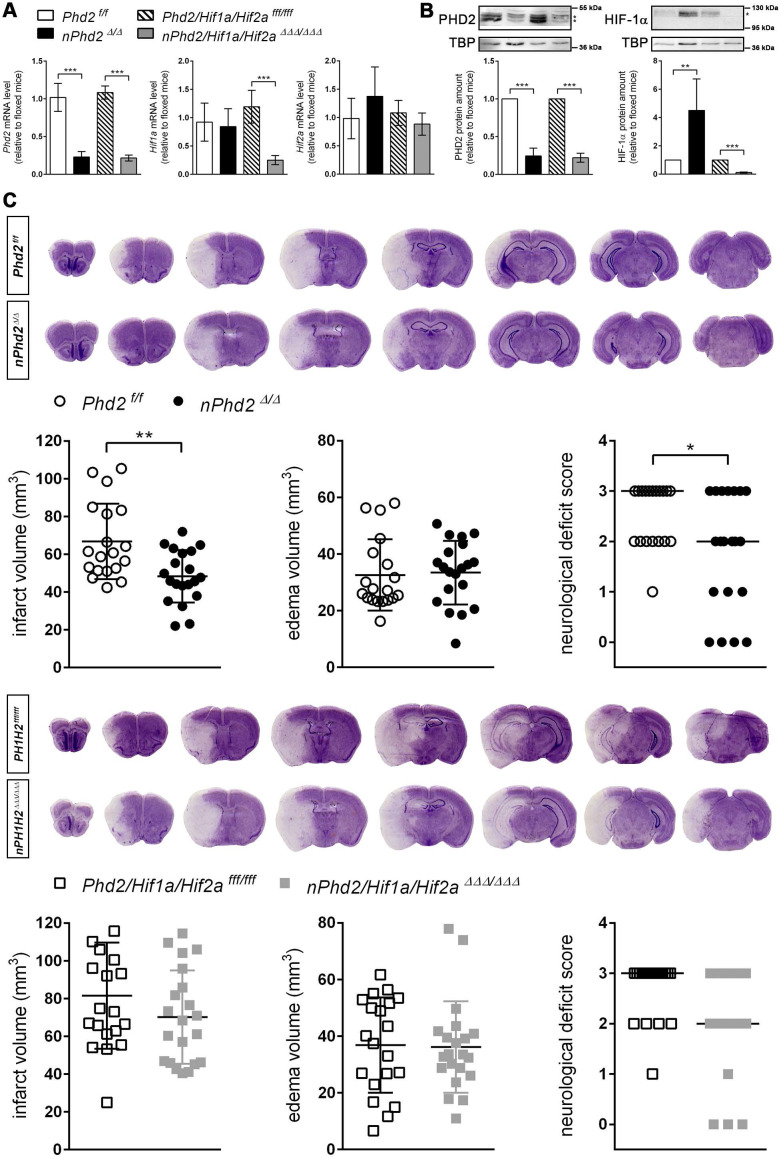
Figure 1.
Neuron-specific PHD2 inactivation reduces brain injury and functional impairment after acute stroke through HIF-dependent mechanisms. (A) RNA and (B) nuclear proteins were prepared from forebrain of adult Phd2 f/f, nPhd2 Δ/Δ, Phd2/Hif1a/Hif2a fff/fff and nPhd2/Hif1a/Hif2a ΔΔΔ/ΔΔΔ mice. (A) Phd2, Hif1a and Hif2a transcript levels were determined by quantitative real-time RT-PCR. Values are normalized to Rps12 and expressed as fold change to Phd2 f/f or Phd2/Hif1a/Hif2a fff/fff, respectively (n = 4-5 per group; unpaired two-tailed Student's t test; *** p < 0.001). (B) Nuclear PHD2 and HIF-α protein levels were quantified by Western blotting (protein bands used for densitometric analysis are marked with an asterisk). Values are normalized to TBP and expressed as fold change to Phd2 f/f or Phd2/Hif1a/Hif2a fff/fff, respectively (n = 4-5 per group; unpaired two-tailed Student's t test; ** p < 0.01, *** p < 0.001). (C) Mice underwent 60 min of MCAO followed by 24 h reperfusion. Infarct and edema volume were determined by cresyl violet staining (n = 19-21 per group; unpaired two-tailed Student's t test; * p < 0.05, ** p < 0.01). Neurological function was assessed using the Bederson neurological deficit score (median; n = 19-21 per group; Mann-Whitney U rank-sum test; * p < 0.05).