Abstract
Objectives
Maropitant is commonly used for acute vomiting. A pharmacokinetic and toxicity study in cats indicated that longer term usage appears safe. The aim of this study was to assess the efficacy of maropitant for management of chronic vomiting and inappetence associated with feline chronic kidney disease (CKD).
Methods
Forty-one cats with stable International Renal Interest Society Stage II or III CKD, no known concurrent illness, and a complaint of chronic vomiting and inappetence attributed to CKD were enrolled in a randomized, placebo-controlled, blinded clinical study. A complete blood count, serum biochemistry, urinalysis, urine culture, T4 and blood pressure were required for entry. Maropitant was administered at a dose of 4 mg orally (median 1.1 mg/kg, range 0.6–2.9 mg/kg) daily for 2 weeks. Owners kept daily logs of vomiting incidence, appetite and activity scores. Physical examination, weight, body condition score and serum biochemistry were performed before and after the trial period. Mann–Whitney statistics were used to compare treatment groups.
Results
Thirty-three cats successfully completed the trial: 21 cats received the drug (nine Stage II cats, 12 Stage III cats) and 12 cats received placebo (seven Stage II cats, five Stage III cats). There was a statistically significant decrease in vomiting in cats with CKD that received maropitant (P <0.01). Cats that received maropitant did not have statistically significant differences in appetite scores, activity scores, weight or serum creatinine compared with placebo.
Conclusions and relevance
Maropitant was demonstrated to palliate vomiting associated with CKD, and may be helpful in the nutritional management of cats with CKD.
Introduction
Chronic kidney disease (CKD) is a common condition in elderly cats, with at least 50% of the population affected. 1 As renal transplantation is often not feasible for owners, medical management is the mainstay of treatment and can help patients live with the metabolic complications of the disease and improve quality of life.2,3 Clinical signs of feline CKD include polyuria, polydipsia, lethargy, decreased appetite, weight loss and vomiting. 4 In the chronically ill patient, and specifically in CKD, poor body condition has been correlated with decreased survival.5–7 Therefore, nutrition is important for long-term prognosis, and treatments that directly target nausea and appetite, in addition to medical therapies for metabolic complications, are of benefit to patients. 8 Several studies have documented the therapeutic value of specially formulated diets in the management of CKD.9–11 However, patients that refuse to eat specially formulated diets cannot benefit from them; therefore, a key therapeutic target for these patients should be the maintenance of appetite and food intake. Additionally, poor appetite is perceived by owners as a significant quality-of-life concern, and anorexia in companion animals can cause emotional distress to owners. 12
The exact pathophysiology of why cats with CKD suffer from anorexia and vomiting is not currently known. A recent study demonstrated that the incidence of classic uremic gastropathy lesions (gastritis and ulceration) appears to be much lower in cats than in other species. 13 Although hypergastrinemia has been documented in cats with CKD, 14 a correlation with gastric hyperacidity has not been demonstrated in cats or humans with CKD.13,15 Without knowing the degree to which uremic gastric lesions or gastric hyperacidity are responsible for clinical signs, other mechanisms should be considered. Uremic toxins are sensed by the chemoreceptor trigger zone of the area postrema, and ablation of this area has been shown to relieve uremic vomiting in dogs. 16
Maropitant (Cerenia; Zoetis) is a selective neurokinin-1 (NK-1) receptor antagonist acting to block effectively the binding of emetic-eliciting substance P, the most potent tachykinin at the NK-1 receptor. 17 Maropitant has been shown to be an effective antiemetic in cats, ameliorating xylazine-induced vomiting when given orally, subcutaneously or intravenously. 17 As xylazine-induced vomiting is mediated by the area postrema in cats, 18 it can be postulated that maropitant would be effective for uremic vomiting. The purpose of this clinical trial was to assess the efficacy of maropitant as an appetite stimulant and an antiemetic in cats with CKD, thus providing support for the use of this medication in the nutritional management of cats with CKD.
Materials and methods
Cats
This was a double-blind, placebo-controlled, multi-institute prospective study. Cats with stable CKD (a priori determination of serum creatinine of 177–442 μmol/l [2.0–5.0 mg/dl]) and a history of decreased appetite, vomiting or poor body condition were enrolled. Diagnostic tests required within 1 month of enrollment included a serum biochemistry profile, complete blood count, urinalysis, urine culture, blood pressure and serum total thyroxine measurement. Exclusion criteria included a normal appetite, other systemic illnesses, complications of CKD (such as pyelonephritis or ureteral obstruction), or history of uremic crisis requiring hospitalization and intravenous fluid therapy within the month prior to study enrollment. Other concurrent therapies such as dietary management, famotidine, potassium supplementation, antihypertensive medications and subcutaneous fluids were accepted therapies if they were started more than 2 weeks before the beginning of the trial and given consistently throughout the study period. No treatment changes were allowed during the study period; if treatment changes were deemed medically necessary, the cat was removed from the study. The study was approved by the Institutional Animal Care and Use Committee at Colorado State University (#11-2556A), and all owners reviewed and signed consent forms prior to participation in the study.
Drug preparation
To allow for a blinded study, commercially available 16 mg maropitant tablets were compounded into 4 mg doses by the pharmacy at the Colorado State University Veterinary Medical Center according to the Professional Compounding Centers of America protocol. The method used is guaranteed to produce accurate compounding to within 10% of the target dose. Analysis of random compounded capsules for maropitant content using liquid chromatography coupled to tandem mass spectrometry showed accuracies of 98.5% ± 1.1% to the intended content and a stability of at least 6 months as formulated. Maropitant capsules were compounded within 6 months of use and stored at room temperature. An identical placebo capsule was manufactured containing lactose. The lots were coded A and B, and the pharmacy staff kept the key to the code until the completion of the study.
Study design
Predetermined randomization for order of treatment allocation, stratified by International Renal Interest Society (IRIS) Stage, was established using an online random number generator, and as cats were enrolled they were assigned consecutively to a treatment group. The clinician and owner were blinded as to the treatment group. A physical examination, body weight measurement, body condition score (BCS; Purina Body Condition System) 19 and serum biochemistry profile were performed at the beginning of the study. The 4 mg or placebo capsule was administered orally daily, followed by 3 ml of water via syringe, for 2 weeks. Owners were asked to fill out a daily log sheet regarding appetite, vomiting episodes, activity level, quality of life and occurrence of unusual behavior in the cats compared with baseline (Supplementary material). At the end of the study period, the log sheets were collected and a physical examination, body weight measurement, BCS and serum biochemistry profile were performed. For each cat enrolled, physical examinations and BCSs were performed by the same clinician. Occurrence of adverse effects was determined using incidence of unusual behaviors recorded in the daily owner log, and results of serum biochemistry performed before and after each phase of the study. All serum biochemistry profiles were performed at the Colorado State University’s diagnostic laboratories.
Statistical analysis
An a priori power calculation was performed based on weight gain as a primary endpoint. Assuming a mean body weight of 3 kg for cats enrolled in the study, a gain in weight of 300 g (0.3 kg) would be regarded as clinically significant. A power calculation was performed using a statistical calculator (Lenth, R. V. [2006]; Java Applets for Power and Sample Size [http://www.stat.uiowa.edu/~rlenth/Power]). Using a difference in means of 0.3 kg, a SD of 0.5 and a P value of 0.05, the power calculation for a paired t-test with 24 cats in the treatment group gives a power of 0.80 for this experiment.
Appetite and activity data were converted to clinical scores; decreased appetite or activity were scored as −1, unchanged appetite or activity were scored as 0 and increased appetite or activity were scored as 1. Scores were then summed for the 2 week treatment period. The number of vomiting episodes over 14 days and summed clinical scores for appetite and activity were compared between placebo and maropitant treatment phases using a Mann–Whitney U-test. Weight and clinicopathologic parameters (serum creatinine, blood urea nitrogen [BUN], phosphorus, potassium) were compared between placebo and maropitant treatment phases at baseline and post-treatment using a Mann–Whitney U-test. For all analyses, a P value of <0.05 was considered to be statistically significant.
Results
Cats
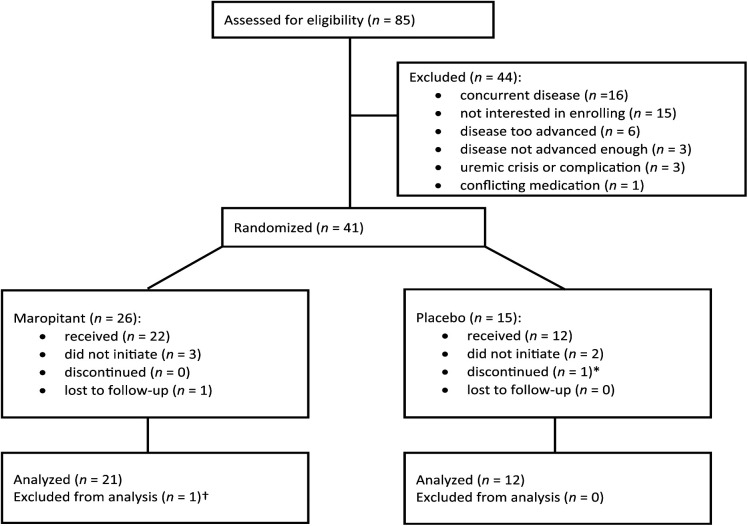
A flow chart outlining study enrollment, allocation, outcome and analysis is depicted in Figure 1. Thirty-four cats successfully completed the trial (one cat was excluded from analysis as it was subsequently discovered to be the other cat in the household that was vomiting); data were analyzed from 21 cats that received drug (nine Stage II cats, 12 Stage III cats) and 12 cats that received placebo (seven Stage II cats, five Stage III cats). The 21 cats allocated to the maropitant group included domestic shorthair (n = 10), domestic longhair (n = 6), domestic mediumhair (n = 1), Siamese (n = 3) and Birman (n = 1). The median age was 14 years (range 10–20 years) with 14 spayed females and seven neutered males. The 12 cats allocated to the placebo group included domestic shorthair (n = 8), domestic longhair (n = 2), Siamese (n = 1) and Ragdoll (n = 1). The median age was 14 years (range 6–18 years) with seven spayed females and five neutered males. There was no statistically significant difference in age or sex between groups. There was no statistically significant difference in weight, serum creatinine, potassium, phosphorus or BUN between maropitant and placebo treatment groups in the prestudy period.
Figure 1.
Flow chart describing assessment for eligibility, enrollment, allocation, outcome and analysis of cats with chronic kidney disease for the maropitant study, which was a placebo-controlled, blinded clinical trial. *Medication was discontinued as the owner could no longer successfully administer it. †Subsequently discovered to be the other cat in the household that was vomiting
The 21 cats allocated to the maropitant group had been receiving a variety of medications, including no medications (n = 7), subcutaneous (SC) fluids (n = 5), benazepril (n = 4), meloxicam (n = 3), mirtazapine (n = 2), amlodipine (n = 2), famotidine (n = 1), aluminum hydroxide (n = 1), potassium supplementation (n = 1), lanthum carbonate (n = 1), polysulfated glycosaminoglycans injection (n = 1), multivitamin (n = 1), ciclosporin (n = 1), butorphanol (n = 1) and tea pill supplement (n = 1). The 12 cats allocated to the placebo group had been receiving a variety of medications, including no medications (n = 6), SC fluids (n = 2), Azodyl renal support supplement (Vetoquinol) (n = 1), amlodipine (n = 1), tramadol (n = 1), amitriptyline (n = 1) and benazepril (n = 1). The 21 cats allocated to the maropitant group had been eating a renal diet (n = 4), a renal diet plus a non-renal diet (n = 9) or a non-renal diet (n = 7). The 12 cats allocated to the placebo group had been eating a renal diet (n = 2), a renal diet plus a non-renal diet (n = 5) or a non-renal diet (n = 5).
No reported changes in medication or diet were made during the study period. At initial assessment for study entry, two cats were treated for a urinary tract infection, one cat was started on amlodipine for hypertension and one cat was started on potassium supplementation. All four cats were required to wait to start the clinical trial an additional 2 weeks beyond the recheck, at which they were deemed medically stable by the attending veterinarian (antibiotics finished and urinary tract cleared as confirmed by urinalysis and culture; blood pressure within normal range; potassium within normal range).
Effect of maropitant in cats with CKD
Administration of maropitant to cats with CKD for 2 weeks resulted in a statistically significant decrease in vomiting in comparison with placebo based on a Mann–Whitney U-test (P <0.01; Figure 2). The median number of vomiting episodes for cats in the maropitant group was zero (range 0–5) and the median number of vomiting episodes for cats in the placebo group was two (range 0–4). There was no statistically significant difference in appetite or activity scores between maropitant and placebo treatment groups during the study period. There was no statistically significant difference in weight, serum creatinine, potassium, phosphorus or BUN between maropitant and placebo treatment groups in the post-study period. Weight and clinicopathologic data are presented in Table 1. No unusual behavior that could be considered an adverse effect was reported in the daily log by the owners.
Figure 2.
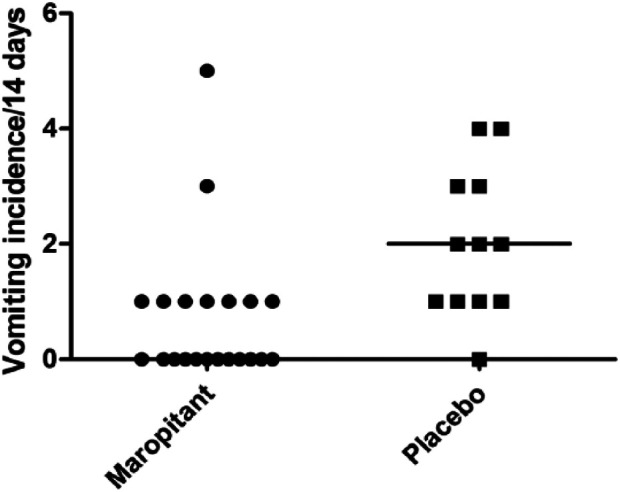
Effect of 2 weeks of maropitant administration on vomiting in cats with chronic kidney disease (CKD). Compared with placebo, a statistically significant decrease in vomiting was seen in cats with CKD (n = 21) administered 4 mg maropitant orally daily for 2 weeks (P <0.01)
Table 1.
Pre- and post-treatment comparison of weight and serum biochemistry parameters relevant to renal function in cats treated with maropitant (4 mg PO daily) or placebo for 14 days. Results are displayed as median (range)
| Maropitant |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pretreatment |
Post-treatment |
Pretreatment |
Post-treatment |
|||||
| Median | Range | Median | Range | Median | Range | Median | Range | |
| Weight (kg) | 3.6 | 2.3–6.0 | 3.6 | 2.3–6.1 | 3.6 | 1.4–6.3 | 3.7 | 1.3–6.3 |
| Creatinine | 3.1 | 2.0–4.0 | 2.7 | 1.9–4.2 | 2.5 | 2.0–4.8 | 2.3 | 1.9–5.3 |
| (0.8–2.4 mg/dl) | ||||||||
| Creatinine | 274 | 177–354 | 239 | 168–371 | 221 | 177–424 | 203 | 168–469 |
| (71–212 μmol/l) | ||||||||
| BUN | 54 | 29–120 | 56 | 31–109 | 52 | 32–77 | 49 | 25–87 |
| (18–35 mg/dl) | ||||||||
| BUN | 19.3 | 10.4–42.8 | 20 | 11.1–38.9 | 18.6 | 11.4–27.5 | 17.5 | 8.9–31.1 |
| (6.4–12.5 μmol/l) | ||||||||
| Phosphorus | 4.7 | 2.8–9.0 | 4.8 | 2.9–9.0 | 4.9 | 2.8–10.2 | 5.2 | 3.0–11.9 |
| (3–6 mg/dl) | ||||||||
| Phosphorus | 1.5 | 0.9–2.9 | 1.6 | 0.9–2.9 | 1.6 | 0.9–3.3 | 1.7 | 1.0–3.8 |
| (1–1.9 μmol/l) | ||||||||
| Potassium | 4.4 | 3.5–5.5 | 4.3 | 3.6–5.8 | 4.4 | 3.6–6.0 | 4.6 | 3.8–5.2 |
| (3.5–5.2 meq/l) | ||||||||
BUN = blood urea nitrogen
Discussion
In this randomized, placebo-controlled, double-blind prospective clinical trial, daily administration of 4 mg maropitant orally for 2 weeks resulted in a statistically significant decrease in vomiting in cats with CKD. This study assesses the use of maropitant for antiemetic therapy in a chronic disease state. Maropitant seems a logical choice for vomiting associated with uremia as it has previously been shown to be effective against xylazine-induced vomiting in cats, 17 and both xylazine and uremic toxins have been demonstrated to act via the area postrema.16 –18 Management of uremic vomiting in CKD cats may be helpful in preserving fluid balance and calories consumed. Similar to a previous safety study in cats, which assessed daily doses up to 5 mg/kg, no apparent adverse effects of daily maropitant administration were appreciated during the 2 week trial period. 17 No statistically significant increase in appetite, weight or activity was documented during the 2 week trial period. Various explanations for this finding include a placebo effect, the length of the clinical trial, the possibility that decreasing vomiting may not have had a discernible effect on appetite or weight, and multi-cat households may affect accuracy of assessment.
Owner assessment of appetite may have been influenced by a placebo effect, thus altering the ability of this study to assess the efficacy of maropitant to improve appetite in CKD cats. Four of 12 cats in the placebo group were reported to have increased appetite for more than 6/14 days during the trial period. In a previous study looking at the effect of the appetite stimulant mirtazapine on the appetite of cats with CKD, similar appetite scores resulted in significant weight gain over 3 weeks. 8 In the four cats in the present study a discernible change in weight was not documented. However, unlike mirtazapine, maropitant has not been described as an appetite stimulant, rather as an antiemetic. Therefore, an increase in appetite is not necessarily expected. An effect on appetite would potentially come secondary to the palliation of nausea associated with uremia, which is an endpoint virtually impossible to measure in veterinary patients. As the effect of maropitant on appetite is potentially more subtle than a drug that acts directly as an appetite stimulant, a 2 week clinical trial may not have been long enough to appreciate weight gain as a result of administration.
During the enrollment phase of the clinical trial, every effort was made to recruit cats that did not have evidence of other systemic disease, particularly one that would cause vomiting. To the best of the attending clinician’s knowledge, this was the case. Abdominal ultrasound and intestinal biopsies would have been helpful to more completely assess enrolled cats but were not possible within the scope of this project. Owing to the commonality of comorbidities in elderly cats, it is therefore possible that cats were enrolled with underlying systemic disease that could have affected their outcomes. One cat in the maropitant group had a vomiting incidence much greater than the rest of the group despite medication administration; vomiting incidence was essentially unchanged during the study period despite medication administration. As this is an unexpected finding given the apparent potency of this drug as an antiemetic, regurgitation misclassified and reported by the owner as vomiting or other unknown systemic disease should be considered as a differential for this cat. A limitation of any feline clinical trial is the likelihood of a multi-cat household making the assessment of food intake and vomiting incidence challenging. Although other members of the feline household could have been vomiting concurrently, most owners felt that they could differentiate their cats’ vomit. However, in one case a cat was removed from analysis when it was subsequently discovered it was in fact the other cat in the household that was chronically vomiting and not the cat with CKD that was enrolled in the study.
One aspect of the study that could potentially confound results is the variety of concurrent therapies that cats were receiving at the time of enrollment. Cats within the maropitant group were collectively receiving more therapies than cats in the placebo group. It is also impossible to say whether a given therapy could have an unknown synergistic effect with maropitant. Unfortunately, it is virtually impossible to standardize therapy without affecting enrollment for clinical trials; thus, the results of the study need to be interpreted with this variability in mind. Another aspect of the study that could affect interpretation of the results is that it cannot entirely be ruled out that an inherent difference in vomiting incidence existed between the treatment groups at baseline. However, as the study was randomized and sufficiently powered it is most likely that the significant difference in vomiting was the effect of maropitant administration.
A limitation of this study was that it was necessary to compound the maropitant in order to blind the study. Because of the concern for accuracy of the dose with compounding, analysis of compounded capsules was performed and found to be acceptable. As owners would typically be administering a quarter of a 16 mg tablet for a cat, dose variability would likely be similar in the clinical setting. Although the dose administered was found to be accurate, there is a possibility the stability of the drug was affected and this could have an unknown effect on the efficacy of the drug.
Conclusions
Daily oral administration of maropitant was demonstrated to palliate vomiting associated with CKD. However, within the 2 week trial period it did not appear to improve significantly appetite or result in weight gain in cats with Stage II and III CKD. However, longer clinical trials and studies in late-stage disease are needed to better assess the effect of maropitant on weight gain and nutrition in cats with CKD.
Supplemental Material
Daily log completed by owners during the study
Acknowledgments
We are grateful to practitioners who assisted with patient enrollment, the Veterinary Information Network for assistance with case recruitment, Dr David Twedt for coordinating study sponsorship, Candice Hastings for assistance with shipping, and Dr Dan Gustafson and Paul Lunghofer of the Pharmacology Core Laboratory at Colorado State University for analysis of maropitant capsules.
Footnotes
Supplementary material: Daily log completed by owners during the study.
The authors have no conflict of interest to declare.
Funding: This study was funded by a non-restricted grant from Zoetis. The sponsor had no role in the study design, collection, analysis and interpretation of data, or preparation of the manuscript for submission. Analysis of maropitant capsules was funded by the Angelo Fund for Feline Therapeutics.
Accepted: 20 September 2014
References
- 1. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2013; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polzin DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 15–30. [DOI] [PubMed] [Google Scholar]
- 3. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42: 669–692. [DOI] [PubMed] [Google Scholar]
- 4. Reynolds BS, Lefebvre HP. Feline CKD: pathophysiology and risk factors – what do we know? J Feline Med Surg 2013; 15 Suppl 1: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis 1994; 24: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 6. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med 2011; 25: 1306–1311. [DOI] [PubMed] [Google Scholar]
- 7. Sanderson BJ. Management of anorexia. In: Bonagura J. (ed). Current veterinary therapy XIII. Philadelphia, PA: WB Saunders, 2000, pp 69–74. [Google Scholar]
- 8. Quimby JM, Lunn KF. Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. Vet J 2013; 197: 651–655. [DOI] [PubMed] [Google Scholar]
- 9. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 10. Plantinga EA, Everts H, Kastelein AM, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec 2005; 157: 185–187. [DOI] [PubMed] [Google Scholar]
- 11. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–957. [DOI] [PubMed] [Google Scholar]
- 12. Reynolds CA, Oyama MA, Rush JE, et al. Perceptions of quality of life and priorities of owners of cats with heart disease. J Vet Intern Med 2010; 24: 1421–1426. [DOI] [PubMed] [Google Scholar]
- 13. McLeland SM, Lunn KF, Duncan CG, et al. Relationship among serum creatinine, serum gastrin, calcium-phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldstein RE, Marks SL, Kass PH, et al. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998; 213: 826–828. [PubMed] [Google Scholar]
- 15. Watanabe H, Hiraishi H, Ishida M, et al. Pathophysiology of gastric acid secretion in patients with chronic renal failure: influence of Helicobacter pylori infection. J Intern Med 2003; 254: 439–446. [DOI] [PubMed] [Google Scholar]
- 16. Borison HL, Hebertson LM. Role of medullary emetic chemoreceptor trigger zone (CT zone) in postnephrectomy vomiting in dogs. Am J Physiol 1959; 197: 850–852. [DOI] [PubMed] [Google Scholar]
- 17. Hickman MA, Cox SR, Mahabir S, et al. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther 2008; 31: 220–229. [DOI] [PubMed] [Google Scholar]
- 18. Colby ED, McCarthy LE, Borison HL. Emetic action of xylazine on the chemoreceptor trigger zone for vomiting in cats. J Vet Pharmacol Ther 1981; 4: 93–96. [DOI] [PubMed] [Google Scholar]
- 19. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Daily log completed by owners during the study