Abstract
This study aimed to investigate the antagonistic effects of a fixed dose of atipamezole (ATI), flumazenil (FLU) and 4-aminopyridine (4AP), both alone and in various combinations, on key stress-related neurohormonal and metabolic changes induced by medetomidine (MED), midazolam (MID) and ketamine (KET) in healthy cats. Seven cats were used consistently in eight investigation groups. Cats were administered a mixture of 0.05 mg/kg MED and 0.5 mg/kg MID followed 10 mins later by 10 mg/kg KET intramuscularly. Twenty minutes after KET injection, the cats were intravenously injected with either a physiological saline solution at 0.1 ml/kg (control) or one of the seven variations of experimental drugs, alone or in combination: ATI, FLU, 4AP, ATI + FLU, FLU + 4AP, ATI + 4AP and ATI + FLU + 4AP. Blood samples were collected 10 times during the 24 h test period. Plasma glucose, insulin, cortisol, epinephrine, norepinephrine and non-esterified fatty acid levels were measured. The administration of MED + MID + KET resulted in hyperglycaemia and decreases in epinephrine, norepinephrine, cortisol and non-esterified fatty acid levels. FLU or 4AP alone or FLU + 4AP did not effectively antagonise the effects induced by MED + MID + KET but enhanced the hyperglycaemia. ATI alone was effective in antagonising these effects. Compared with non-ATI regimens, combinations with ATI were more effective in antagonising the effects induced by MED + MID + KET; however, ATI + FLU + 4AP caused large increases in cortisol, epinephrine and norepinephrine concentrations. ATI, both alone and in combination, is effective in antagonising the neurohormonal and metabolic effects of MED + MID + KET in cats. However, ATI + FLU + 4AP is not suitable because of large stress-related hormonal responses.
Introduction
The α2-adrenoceptor agonist medetomidine (MED) is mainly used for sedation, analgesia and muscle relaxation in veterinary medicine. However, it induces undesirable effects, such as hyperglycaemia, hypoinsulinaemia, emesis, diuresis and bradyarrhythmias, in dogs and cats.1 –3 Our previous study demonstrated that a combination of MED, the benzodiazepine agonist midazolam (MID) and the dissociative anaesthetic agent ketamine (KET) produced good anaesthesia in cats, with excellent muscle relaxation and analgesia. 4 Antagonism may be required when anaesthetised animals show a profound depression of vital signs, adverse effects and/or delayed recovery from anaesthesia. Atipamezole (ATI), flumazenil (FLU) and 4-aminopyridine (4AP) completely or partially antagonise the effects of MED, MID and KET, respectively, in cats.5 –9 These antagonists are beneficial in the clinic setting when utilising MED + MID + KET anaesthesia, and are useful in the support of emergency and critical care associated with anaesthesia. We previously evaluated the antagonistic effects of intravenously administered ATI, FLU and 4AP (alone and in various combinations) for anaesthesia produced by a fixed dose of MED + MID + KET injected intramuscularly in cats. 4 The combination of ATI + FLU + 4AP was the most effective in antagonising the anaesthesia induced by MED + MID + KET; however, it was unsuitable for a smooth recovery from anaesthesia because the triple antagonist regimen was associated with clinical manifestations such as tachycardia, tachypnoea, excitement and muscle tremors. 4 Whether these symptoms constitute stressful events associated with the use of the combination of ATI + FLU + 4AP remains unclear. Stress-related hormonal responses associated with this combination may provide useful information.
Stress consists of the biological responses of an animal in an attempt to cope with a disruption or threat to homeostasis. 10 Stressors such as anxiety, excitement, pain, anaesthesia and other factors are known to induce neurohormonal and metabolic changes in animals. 11 These changes are characterised by increases in blood levels of cortisol, catecholamines, glucose and non-esterified fatty acids (NEFAs) and a decrease in blood insulin levels. 11 Actions mediated by α2-adrenoceptors are closely coordinated with these events. In dogs and cats, MED has been reported to suppress catecholamine release, insulin release and lipolysis, and it has been reported to induce hyperglycaemia; these changes have been found to be reversed by ATI.1,2,12 In addition, KET has been reported to increase plasma catecholamine and cortisol levels in dogs. 13 However, no data are available on the neurohormonal and metabolic effects of MED + MID + KET anaesthesia in cats. In addition, it is important to examine the stress-related neurohormonal and metabolic responses of ATI, FLU and 4AP to ensure the appropriate use of antagonistic agents during the use of MED + MID + KET anaesthesia in cats. The present study aimed to investigate the effects of ATI, FLU and 4AP, both alone and in various combinations, on key stress-related neurohormonal and metabolic variables in healthy cats anaesthetised with MED + MID + KET.
Materials and methods
Animals
Seven healthy mixed-breed cats (two intact males, two intact females, two neutered males and one neutered female), with an (mean ± SD) age of 4.0 ± 1.8 years and a weight of 4.2 ± 0.7 kg were used. The cats were housed in the laboratory for at least 1 month before study initiation and were fed standard commercial dry food. Routine haematological examination before the study revealed that all values were within normal physiological ranges. The experimental protocol was approved by the Animal Research Committee of Tottori University, Tottori, Japan.
Experimental protocol
The seven cats were consistently used in each of the eight groups according to a randomised design. There were at least 3 weeks between treatments for each cat. Cats were intramuscularly administered the mixture of 0.05 mg/kg of MED (medetomidine hydrochloride, 1 mg/ml Domitor; Meiji Seika Kaisha) and 0.5 mg/kg of MID (midazolam hydrochloride, 5 mg/ml Dormicum; Astellas Pharma), which was followed by intramuscular administration of 10 mg/kg of KET (ketamine hydrochloride, 50 mg/ml Ketalar; Daiichisannkyo Kaisha) 10 mins later. MED and MID were mixed in the same syringe immediately before injection. Twenty minutes after KET injection, the cats were administered an intravenous dose of either 0.1 ml/kg physiological saline solution (control), 0.2 mg/kg ATI (atipamezole hydrochloride, 5 mg/kg Antisedan; Meiji Seika Kaisha), 0.1 mg/kg FLU (0.1 mg/ml Anexate; Yamanouchi Pharmaceutical), 0.5 mg/kg 4AP (Wako Pure Chemical Industries) or all possible combinations (ATI + FLU, FLU + 4AP, ATI + 4AP or ATI + FLU + 4AP). 4AP was dissolved in the saline solution at a concentration of 2.5 mg/ml. The potential antagonists were mixed in the same syringe immediately before injection and injected into the jugular vein. The cats were fasted for 12 h before the injection of each agent. Food and water were offered again 1 h after the last blood sampling of the day. During the sampling period, the cats were kept in a room with the air temperature set at 25°C.
Instrumentation and sample collection
On the day before the experiment, a 17 G central venous catheter (EXCV catheter kit; Tyco Healthcare Japan) was introduced into the jugular vein under general anaesthesia. Before catheter placement, 6.6–8.8 mg/kg of propofol (Rapinovet; Schering-Plough Animal Health) was intravenously administered until adequate anaesthesia was induced. Anaesthesia was maintained with a constant infusion of 0.22–0.44 mg/kg/min of propofol. The catheter was flushed with 1.5 ml of heparinised physiological saline solution, capped and fixed. The cats were then placed in individual cages to rest overnight. Blood samples (2 ml per sampling) were collected from the catheter prior to the injection of MED + MID (pretest value), immediately before injection of the antagonist agents ATI/FLU/4AP (time 0), as well as at 0.5, 1, 2, 3, 4, 5, 6 and 24 h after injection of the antagonists. After each sampling, the cats were returned to their cages. Packed cell volume and the other routine haematological variables were monitored throughout the sampling period. After each experiment, the catheter was removed and the cats were allowed to recover.
Sample processing and analysis
Blood was mixed with EDTA to prevent clotting. Samples were immediately centrifuged at 4°C; the plasma was then separated and stored at −80°C until analysis for levels of glucose, insulin, cortisol, epinephrine, norepinephrine and NEFAs. Glucose, NEFA, insulin, cortisol and catecholamine levels were measured according to previously published methods.2,14 Glucose and NEFA levels were determined by an enzyme assay using commercially available kits (Glucose CII-test and NEFA C-test, respectively; Wako Pure Chemical) and measured by means of a spectrophotometer. Insulin levels were measured by double-antibody radioimmunoassay with a kit (I-AJ16; Eiken Chemical Company). Cortisol was measured by single-antibody radioimmunoassay using a kit (Gamma Coat Cortisol; Nihon Sheering). Catecholamines were extracted on activated alumina and measured using high-performance liquid chromatography and an electrochemical detector (Coulochem II; ESA). As an internal standard, 3,4-dihydroxybenzylamine was used. The percentage recovery of authentic 3,4-dihydroxybenzylamine was 55–70%. Intra- and inter-assay coefficients of variation and limits of detection and quantification for assays of each variable are shown in Table 1.
Table 1.
Intra- and inter-assay coefficients of variation and limits of detection and quantification for assays of each variable used in this study
| Variable | Method of assay | Coefficients of variation (%) |
Limits of detection |
Limits of quantification | ||
|---|---|---|---|---|---|---|
| Intra-assay | Inter-assay | Lower | Upper | |||
| Glucose | Enzyme | 0.5–2.0 | 1.0–2.5 | 3.8 mg/dl | 700 mg/dl | 15 mg/dl |
| NEFA | Enzyme | 1.2–3.0 | 1.9–3.2 | 0 µEq/l | 2000 µEq/l | 16 µEq/l |
| Insulin | Radioimmunoassay | 2.1–6.8 | 2.4–5.6 | 1 µU/ml | 370 µU/ml | 1.1 µU/ml |
| Cortisol | Radioimmunoassay | 3.5–5.0 | 4.2–8.7 | 0.23 µg/dl | 60 μg/dl | 0.30 μg/dl |
| Epinephrine | HPLC-ECD | 0.5–2.0 | – | 20 pg/ml | – | 20 pg/ml |
| Norepinephrine | HPLC-ECD | 0.2–1.5 | – | 10 pg/ml | – | 11 pg/ml |
NEFA = non-esterified fatty acid; HPLC-ECD = high-performance liquid chromatography and electrochemical detection
Behavioural scoring of recovery
The overall quality of recovery from anaesthesia was assessed using a modification of previously published scoring methods as follows: 15 1 (excellent) = smooth, quiet, comfortable, no stiffness, no shivering and no hypersensitivity to touch; 2 (good) = mild rigidity of either or both of the thoracic and pelvic limbs, mild sensitivity to touch and mild shivering with symptoms persisting transiently; 3 (moderate) = marked rigidity of all four limbs, shivering and hypersensitivity to touch with signs persisting <30 mins; 4 (poor) = more marked demonstration of events mentioned before, with these persisting >30 mins; and 5 (extremely poor) = marked rigidity of all four limbs, marked hypersensitivity to touch and possible aggression with signs persisting >60 mins. The observer scoring the above-mentioned behaviours was blinded to each treatment.
Statistical analysis
All data obtained were analysed using Prism statistical software (version 4; GraphPad Software). ANOVA for repeated measurements was used to examine the time effect on glucose, insulin, cortisol, epinephrine, norepinephrine and NEFA concentrations within each group. When a significant difference was found, the Tukey test was used for multiple comparisons of the means. The area under the curve (AUC) for 0–6 h was calculated for each biochemical variable. AUC was measured by calculating the sum of the trapezoids formed by the data points and the x-axis. The AUC data were tested for normality with Shapiro–Wilk test. When the data were normally distributed, the t-test with Bonferroni correction was used for paired comparisons between the groups. When the AUC data were not normally distributed, the Wilcoxon signed-rank test with Bonferroni correction was used to compare the differences between the groups. In both tests, the significance level was indicated by a P value of <0.00625. The score data were analysed using the Wilcoxon signed-rank test for paired treatment comparisons, and a P value of <0.00625 was considered significant by the Bonferroni correction. For other tests, P values of <0.05 were considered statistically significant.
Results
NEFA
Plasma NEFA concentrations decreased significantly at 0–2 h compared with pretest values in the control and FLU+ 4AP groups, but did not significantly decrease in the groups administered ATI (Figure 1a). The AUC data from 0–6 h for NEFA levels were significantly higher in the ATI + 4AP and ATI + FLU + 4AP groups than the control and FLU groups, and in the ATI + FLU, ATI + 4AP and ATI + FLU + 4AP groups than the FLU + 4AP group (Figure 1b).
Figure 1.
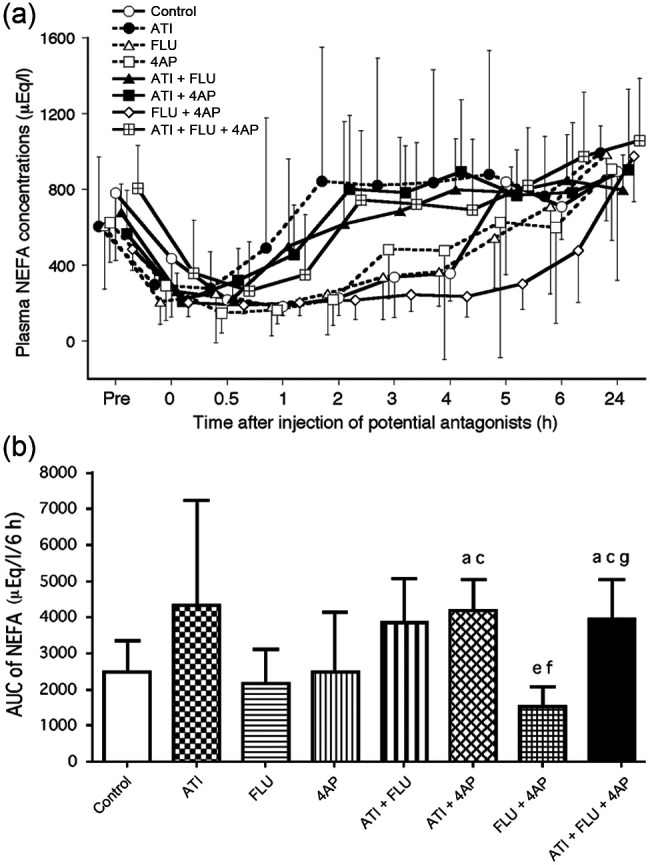
(a) Changes in plasma non-esterified fatty acid (NEFA) concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). Each point and vertical bars show the mean ± SD. (b) Area under the curve (AUC) data from 0–6 h for NEFA values after administration of antagonists. Each vertical bar indicates the mean and SD. a = Significantly different from control; b = significantly different from atipamezole (ATI); c = significantly different from flumazenil (FLU); d = significantly different from 4-aminopyridine (4AP); e = significantly different from ATI + FLU; f = significantly different from ATI + 4AP; g = significantly different from FLU + 4AP. The significance level is P <0.00625
Glucose
Compared with pretest values, plasma glucose concentrations increased significantly at 0.5–2 h in the control group, at 0 h in the ATI and ATI + FLU groups, at 0–0.5 h in the ATI + 4AP and ATI + FLU + 4AP groups and at 0–3.0 h in the FLU, 4AP and FLU + 4AP groups (Figure 2a). The AUC values from 0–6 h were significantly higher in the FLU, 4AP and FLU + 4AP groups than the ATI and ATI + FLU + 4AP groups (Figure 2b).
Figure 2.
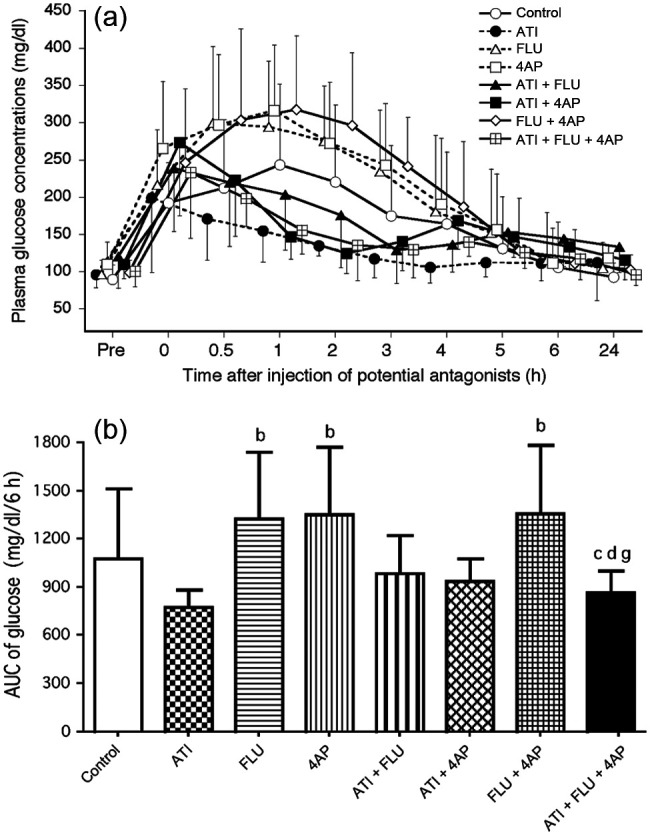
(a) Changes in plasma glucose concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). (b) Area under the curve (AUC) data from 0–6 h for glucose values after administration of antagonists. Plots, abbreviations and footnotes (a–g) are as described in Figure 1
Insulin
Plasma insulin concentrations decreased significantly at 0 and 1 h compared with the baseline in the FLU group (Figure 3a). There were no significant differences in AUC values from 0–6 h between any of the treatment groups (Figure 3b).
Figure 3.
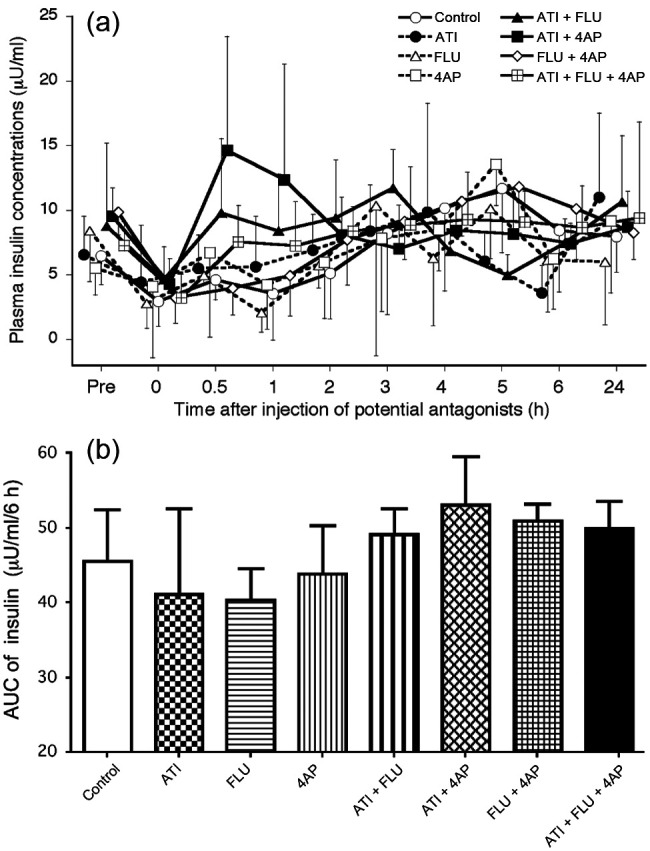
(a) Changes in plasma insulin concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). (b) Area under the curve (AUC) data from 0–6 h for insulin values after administration of antagonists. Plots, abbreviations and footnotes (a–g) are as described in Figure 1
Cortisol
In the ATI + FLU + 4AP group, the cortisol concentrations increased significantly at 3–5 h compared with baseline (Figure 4a). Significant increases in cortisol concentrations were also observed at 3 and 6 h in the ATI + FLU group. The AUC values from 0–6 h were significantly higher in the ATI + FLU + 4AP group than the FLU and FLU + 4AP groups (Figure 4b).
Figure 4.
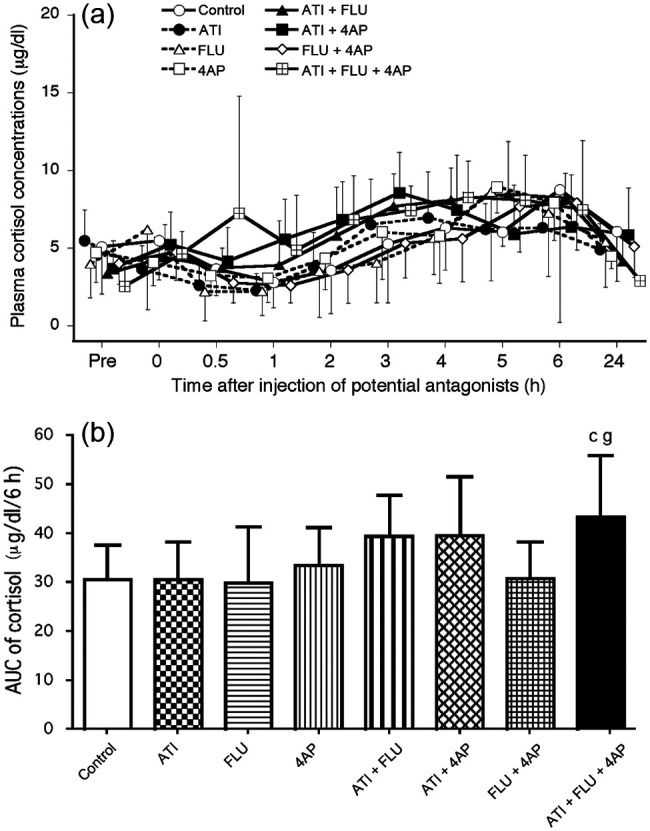
(a) Changes in plasma cortisol concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). (b) Area under the curve (AUC) data from 0–6 h for cortisol values after administration of antagonists. Plots, abbreviations and footnotes (a–g) are as described in Figure 1
Epinephrine
The epinephrine concentrations decreased significantly at 0–5 h compared with the baseline in the control and FLU + 4AP groups, and at 0.5–6 h in the FLU and 4AP groups (Figure 5a). The epinephrine concentrations in the groups administered ATI did not decrease significantly at 0.5–6 h compared with baseline. The AUC values from 0–6 h were significantly higher in the ATI + FLU + 4AP group than in the control and non-ATI groups (Figure 5b).
Figure 5.
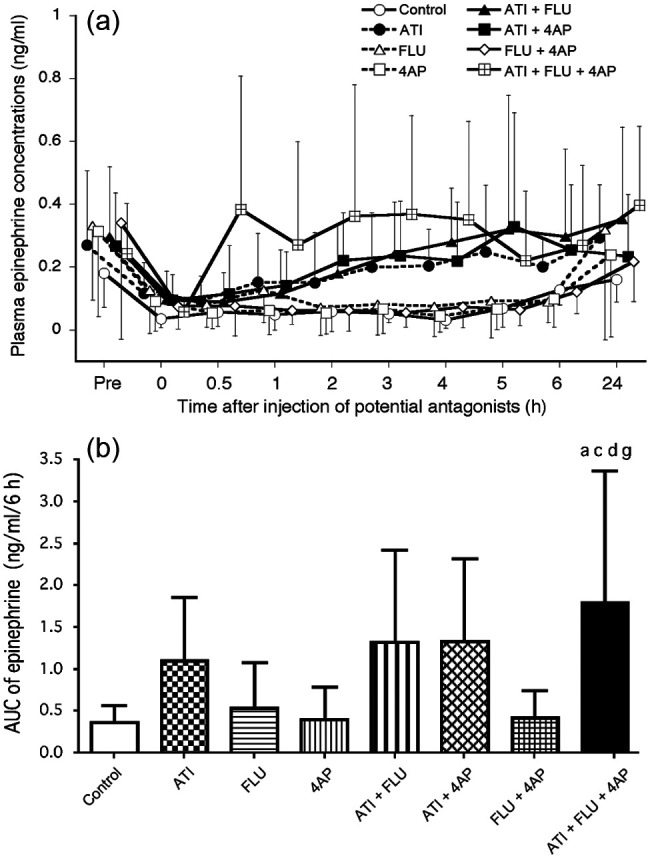
(a) Changes in plasma epinephrine concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). (b) Area under the curve (AUC) data from 0–6 h for epinephrine values after administration of antagonists. Plots, abbreviations and footnotes (a–g) are as described in Figure 1
Norepinephrine
The norepinephrine concentrations decreased significantly at 0.5–4 h compared with the baseline in the control, at 0–5 h in the 4AP and FLU + 4AP groups and at 0–6 h in the FLU group (Figure 6a). The norepinephrine concentrations in the groups administered ATI did not decrease significantly at 0–24 h compared with baseline. However, two cats in the ATI + FLU + 4AP group showed large increases in epinephrine concentrations at 2–4 h compared with the baseline. For example, the concentrations in one cat increased from 1.48–12.57 ng/ml at 3 h, whereas those in the other cat increased from 0.75–7.89 ng/ml at 3 h. The AUC value from 0–6 h was significantly higher in the ATI + FLU + 4AP group than the non-ATI groups, and in the ATI + 4AP group than the FLU group (Figure 6b).
Figure 6.
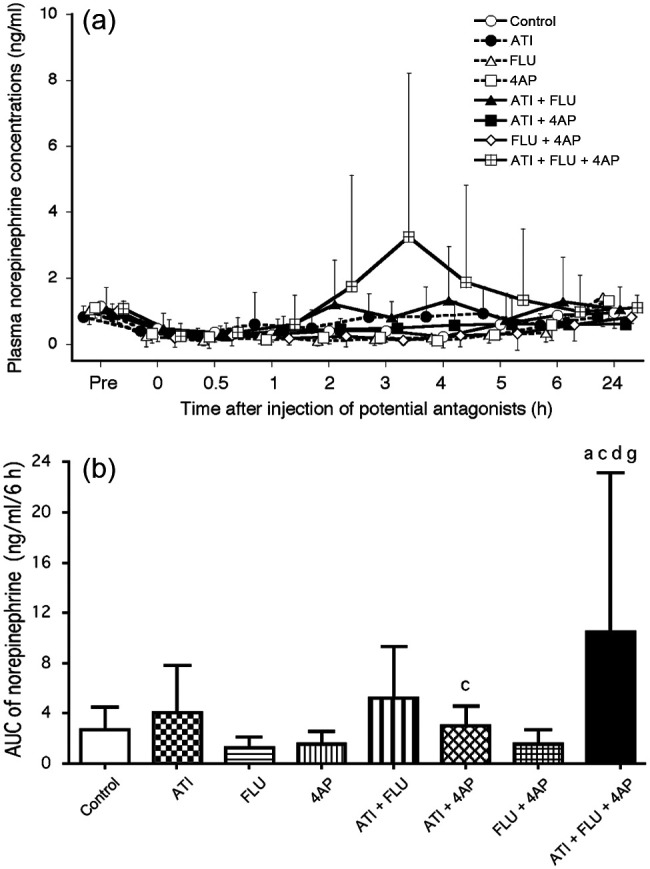
(a) Changes in plasma norepinephrine concentrations after administration of antagonists, either alone or in combination, in seven cats anaesthetised with medetomidine, midazolam and ketamine (MED + MID + KET). (b) Area under the curve (AUC) data from 0–6 h for norepinephrine values after administration of antagonists. Plots, abbreviations and footnotes (a–g) are as described in Figure 1
Recovery score
Behavioural quality during the recovery process from anaesthesia in each group was similar to previously described results. 4 Recovery score data were significantly higher in the ATI + FLU + 4AP group than in the non-ATI groups (Table 2).
Table 2.
Behavioural recovery score after administration of antagonists, either alone or in combination, in cats anaesthetised with medetomidine, midazolam and ketamine
| Group | Recovery score |
|---|---|
| Control | 1 (1–2) |
| ATI | 2 (1–3) |
| FLU | 1 (1–2) |
| 4AP | 1 (1–2) |
| ATI + FLU | 2 (1–3) |
| ATI + 4AP | 2 (1–5) |
| FLU + 4AP | 1 (1–2) |
| ATI + FLU + 4AP | 4 (2–5)* † ‡ § |
Values represent the median (range) of seven cats
Significantly different from control
Significantly different from FLU
Significantly different from 4AP
Significantly different from FLU + 4AP. The significance level is P <0.00625
ATI = atipamezole; FLU = flumazenil; 4AP = 4-aminopyridine
Discussion
The rationale for fixed-dosing of ATI, FLU and 4AP as antagonists for MED + MID + KET anaesthesia was outlined in our earlier study. 4 In the present study, for supportive management during emergencies immediately after the balanced anaesthesia, we selected intravenous administration of antagonists because of the immediate effect (within 20 mins of administration). Alternatively, intramuscular delivery, in comparison with intravenous administration of antagonists, may induce a lower stress response; conducting an intramuscular trial on the stress response in the future may be informative. A total sampling of 20 ml of blood over 24 h, as conducted in this study, may potentially affect the stress response. However, our previous study found that blood sampling using similar procedures did not significantly alter plasma concentrations of glucose, insulin, glucagon, cortisol, NEFAs, norepinephrine or epinephrine in non-medicated normal cats. 14 Therefore, it is conceivable that 2 ml blood sampling repeated 10 times at a 1 h interval over 24 h does not induce apparent effects on stress responses in healthy cats. In this study, the priority for determining the AUC time base of NEFAs, glucose and catecholamines was based on the recovery time for changes that were recorded in the control group. The time period for the cortisol and insulin AUCs was determined to be the same as that for catecholamines and other metabolites as indicators of the stress hormonal response. The measurement at 24 h post-treatment was performed to reconfirm the return to premedication values.
The results of the present study revealed that MED + MID + KET anaesthesia produced a moderate hyperglycaemia, primarily because of the effect of MED, which has previously been reported to induce a dose-dependent and marked hyperglycaemia in cats. 2 This hyperglycaemic effect may limit the use of MED + MID + KET anaesthesia in cats with metabolic and neurohormonal problems such as diabetes mellitus, ketosis and glycosuria. The present results demonstrated that ATI alone or in combination with other drugs reversed hyperglycaemia induced by MED + MID + KET. By contrast, FLU, 4AP and FLU + 4AP did not decrease hyperglycaemia; rather, surprisingly, FLU, 4AP and FLU + 4AP enhanced MED + MID + KET-induced hyperglycaemia. Although the precise mechanism is unknown, it has been reported that feline hyperglycaemia induced by 40 µg/kg MED can be decreased by concomitant administration of 0.5 mg/kg MID. 14 The augmentation effect of FLU treatment on MED + MID + KET-induced hyperglycaemia may be caused by the antagonism of the effect of MID suppressing the hyperglycaemic action of MED. It was also found that 4AP treatment enhanced the hyperglycaemic effect induced by MED + MID + KET. Although the precise mechanism of this effect is also unknown, treatments with FLU and/or 4AP are not clinically recommended for decreasing the hyperglycaemia associated with MED + MID + KET anaesthesia in cats. Therefore, ATI is potentially useful for treating hyperglycaemic problems after MED + MID + KET anaesthesia in cats.
In the present study, the plasma insulin concentration at 1 h after FLU treatment decreased significantly, suggesting that FLU at least partially influenced MED + MID + KET-induced hyperglycaemia. Although plasma insulin concentrations tended to be transiently decreased after MED + MID + KET anaesthesia and reversed by ATI, AUC from 0–6 h for plasma insulin concentration did not significantly differ between any of the treatments, indicating that ATI, FLU and 4AP, both alone and in various combinations, do not have a long-term influence on the plasma insulin concentration after MED + MID + KET anaesthesia in cats.
The changes in NEFA concentrations are clinically significant because they are affected by hormones such as cortisol and catecholamines, which are associated with the stress response. 11 In the present study, plasma NEFA concentrations decreased after MED + MID + KET administration. This change was reversed by treatments with ATI, ATI + FLU and ATI + FLU + 4AP. It has been reported that plasma NEFA concentrations are decreased by MED but that the addition of MID fails to have an effect on this in cats.2,14 However, KET (10 mg/kg) alone fails to alter NEFA concentrations in dogs. 13 Therefore, treatments including ATI are clinically effective in antagonising the reduction in plasma NEFAs induced by MED + MID + KET anaesthesia in cats. The fluctuation of plasma NEFA concentrations may not be directly harmful to the anaesthetised cats; however, it is preferable that this NEFA change during and after anaesthesia is as small as possible because it indirectly reflects the glucose homeostasis and metabolism in adipose tissue and the liver associated with sympathoadrenal activation or inhibition. 16 In this regard, treatments including ATI will be clinically significant as an index of feline metabolic stability.
We have previously shown that MED alone or a combination of MED + MID does not affect the plasma cortisol concentration in cats.2,14 However, premedication with MED has been reported to reduce or delay the increase in plasma cortisol concentrations induced by ovariohysterectomy in dogs.17,18 In dogs, it has been reported that the MED + KET combination prevented this effect despite KET monotreatment increasing plasma cortisol concentrations. 13 In the present study, MED + MID + KET anaesthesia also failed to affect significantly plasma cortisol concentration in cats, similar to ATI, FLU, 4AP and FLU + 4AP administration. However, cortisol release was stimulated by an intervention combination of ATI + FLU + 4AP. The present study also reconfirmed that the recovery from anaesthesia was extremely poor in the ATI + FLU + 4AP group, as described in our previous study. 4 These results suggest that treatment with ATI + FLU + 4AP is not clinically recommended because of the large increase in adrenocortical activity. Furthermore, plasma cortisol concentrations may be more excessively elevated after the antagonism by ATI + FLU + 4AP when surgery such as ovariohysterectomy is added to MED + MID + KET anaesthesia in feline practice.
It has been reported that MED greatly inhibits plasma epinephrine and norepinephrine concentrations in cats and dogs.1,2 In contrast, plasma epinephrine and norepinephrine concentrations have been found to be increased by MID in cats and by KET in dogs.13,14 The KET-induced increases in plasma concentrations of epinephrine and norepinephrine have been reported to be mitigated by MID, 19 and even abolished by MED. 13 In addition, MID has been reported to decrease MED-induced inhibition of norepinephrine release in cats. 14
Ultimately, the present study revealed that the MED + MID + KET combination decreased plasma concentrations of both epinephrine and norepinephrine in cats, suggesting that these effects are mainly attributed to MED. The present study revealed that FLU, 4AP and FLU + 4AP did not significantly alter MED + MID + KET-induced reduction in plasma concentrations of both epinephrine and norepinephrine. However, ATI alone, ATI + FLU, ATI + 4AP and ATI + FLU + 4AP reversed these effects. Furthermore, we found that ATI alone, ATI + FLU and ATI + 4AP had similar effects but that the ATI + FLU + 4AP combination caused large increases in both plasma epinephrine and norepinephrine concentrations during recovery from anaesthesia. In particular, an increase in the norepinephrine concentration was prominent. It has been reported that 4AP can release norepinephrine from the sympathetic ganglion and perivascular nerve endings, as well as being able to enhance the release of acethylcholine.20,21 These actions of 4AP may contribute to prominent elevation of norepinephrine after administration of ATI + FLU + 4AP. These results suggest that treatment with ATI + FLU + 4AP is not clinically recommended because of a large increase in sympathoadrenergic activity. Care must be taken in the further enhancement of sympathoadrenergic activity when the triple antagonist regimen is used for antagonism to MED + MID + KET anaesthesia in cats having cardiovascular problems such as tachycardia. In addition, FLU or 4AP alone is not clinically recommended for reversing the inhibition of catecholamine release induced by MED + MID + KET in cats. However, as an overall effect of the antagonists, the use of potential antagonists may be advantageous for recovery from anaesthesia if the sympathoadrenomedullary system is activated adequately but not excessively.
Conclusions
The results of this study revealed that ATI, both alone and in combination, is effective in antagonising the neurohormonal and metabolic effects of MED + MID + KET in cats. The use of 4AP and FLU is not clinically recommended in the antagonism of the hormonal and metabolic effects induced by MED + MID + KET, even if they are effective in hastening recovery from anaesthesia. 4 It is also reported that the addition of FLU to ATI alone does not produce a significant difference in the recovery time and total time of immobilisation by MED + MID + KET anaesthesia in cats, although excitation and hyperaesthesia are not observed in the ATI + FLU combination. 22 ATI alone may give appropriate antagonism without large stress responses for the recovery from anaesthesia. However, the triple combination (ATI + FLU + 4AP) is not suitable for smooth antagonism because of large stress-related hormonal responses, as well as poor recovery from anaesthesia, including hypersensitivity and aggression.
Acknowledgments
We wish to thank Dr T Ueoka of the Ueoka Animal Hospital for technical assistance.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This work was supported, in part, by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to Yoshiaki Hikasa).
Accepted: 29 September 2014
References
- 1. Ambrisko T, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res 2002; 66: 42–49. [PMC free article] [PubMed] [Google Scholar]
- 2. Kanda T, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in healthy cats. Can J Vet Res 2008; 72: 278–286. [PMC free article] [PubMed] [Google Scholar]
- 3. Murahata Y, Hikasa Y. Comparison of the diuretic effects of medetomidine hydrochloride and xylazine hydrochloride in healthy cats. Am J Vet Res 2012; 73: 1871–1880. [DOI] [PubMed] [Google Scholar]
- 4. Ueoka N, Hikasa Y. Antagonistic effects of atipamezole, flumazenil and 4-aminopyridine against anaesthesia with medetomidine, midazolam and ketamine combination in cats. J Feline Med Surg 2008; 10: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savola JM. Cardiovascular action of medetomidine and their reversal by atipamezole. Acta Vet Scand 1989; 85: 38–47. [PubMed] [Google Scholar]
- 6. Vähä-Vahe T. Clinical effectiveness of atipamezole as a medetomidine antagonist in cats. J Small Anim Pract 1990; 31: 193–197. [DOI] [PubMed] [Google Scholar]
- 7. Granholm M, McKusick BC, Westerholm FC, et al. Evaluation of the clinical efficacy and safety of dexmedetomidine or medetomidine in cats and their reversal with atipamezole. Vet Anaesth Analg 2006; 33: 214–223. [DOI] [PubMed] [Google Scholar]
- 8. Ilkiw JE, Farver TB, Suter CM, et al. The effect of intravenous administration of variable-dose flumazenil after fixed-dose ketamine and midazolam in healthy cats. J Vet Pharmacol Ther 2002; 25: 181–188. [DOI] [PubMed] [Google Scholar]
- 9. Hatch RC, Booth MH, Kitzman JV, et al. Antagonism of ketamine anaesthesia in cats by 4-aminopyridine and yohimbine. Am J Vet Res 1983; 44: 417–423. [PubMed] [Google Scholar]
- 10. Gaynor JS, Muir WW. Handbook of veterinary pain management. 1st ed. St Louis, MO: Mosby Year Book, 2002, p 49. [Google Scholar]
- 11. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000; 85: 109–117. [DOI] [PubMed] [Google Scholar]
- 12. Ambrisko TD, Hikasa Y. The antagonistic effects of atipamezole and yohimbine on stress-related neurohormonal and metabolic responses induced by medetomidine in dogs. Can J Vet Res 2003; 67: 64–67. [PMC free article] [PubMed] [Google Scholar]
- 13. Ambrisko TD, Hikasa Y, Sato K. Influence of medetomidine on stress-related neurohormonal and metabolic effects caused by butorphanol, fentanyl, and ketamine administration in dogs. Am J Vet Res 2005; 66: 406–412. [DOI] [PubMed] [Google Scholar]
- 14. Kanda T, Hikasa Y. Effects of medetomidine and midazolam alone or in combination on the metabolic and neurohormonal responses in healthy cats. Can J Vet Res 2008; 72: 332–339. [PMC free article] [PubMed] [Google Scholar]
- 15. Zaki S, Ticehurst K, Miyaki Y. Clinical evaluation of Alfaxan-CD® as an intravenous anaesthetic in young cats. Aust Vet J 2009; 87: 82–87. [DOI] [PubMed] [Google Scholar]
- 16. Fagerholm V, Haaparanta M, Scheinin M. α2-Adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol 2011; 108: 365–370. [DOI] [PubMed] [Google Scholar]
- 17. Benson GJ, Grubb TL, Neff-Davis C, et al. Perioperative stress response in the dog: effect of pre-emptive administration of medetomidine. Vet Surg 2000; 29: 85–91. [DOI] [PubMed] [Google Scholar]
- 18. Ko JC, Mandsager RE, Lange DN, et al. Cardiorespiratory responses and plasma cortisol concentrations in dogs treated with medetomidine before undergoing ovariohysterectomy. J Am Vet Med Assoc 2000; 217: 509–514. [DOI] [PubMed] [Google Scholar]
- 19. Adams HA. Endocrine reactions following S-(+)-ketamine. Anaesthesist 1997; 46 Suppl 1: S30–S37. [DOI] [PubMed] [Google Scholar]
- 20. Durant NN, Lee C, Katz RL. 4-Aminopyridine reversal of sympathetic ganglionic blockade in the anaesthetised cat. Anesthesiology 1980; 52: 381–384. [DOI] [PubMed] [Google Scholar]
- 21. Kun A, Pataricza J, Krassói I, et al. Low 4-aminopyridine concentration-induced contraction is mediated by neuronal noradrenaline in canine saphenous vein. Vascul Pharmacol 2002; 39: 7–11. [DOI] [PubMed] [Google Scholar]
- 22. Ebner J, Wehr U, Baumgartner C, et al. Partial antagonization of midazolam-medetomidine-ketamine in cats – atipamezole versus combined atipamezole and flumazenil. J Vet Med A Physiol Pathol Clin Med 2007; 54: 518–521. [DOI] [PubMed] [Google Scholar]


