Abstract
Objectives
The purpose of this study was to evaluate the effect of sudden alterations in heart rate (HR) on left ventricular (LV) wall thickness and dimensions determined by echocardiography in healthy cats.
Methods
Six experimental cats were used. All cats were anaesthetised and HR was controlled with right atrial pacing. The interventricular septum and left ventricular free wall thickness at end diastole (IVSd and LVFWd, respectively), left ventricular end-diastolic and end-systolic diameter (LVIDd and LVIDs, respectively) and shortening fraction (FS) of each cat were assessed using echocardiography at pacing rates of 120, 130, 140, 150, 160, 170 and 180 ppm.
Results
There were significant relationships between HR and IVSd, LVFWd, LVIDd, LVIDs and FS. As the HR increased, LV wall thickness increased and chamber dimensions got smaller in a linear fashion. The maximum and minimum differences in wall thickness between 120 ppm and 180 ppm were 2.0 mm and 0.7 mm in single measurements, respectively.
Conclusions and relevance
LV wall thickness and dimensions were significantly influenced by alterations in HR.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common heart disease in cats.1,2 HCM is characterised by concentric left ventricular (LV) hypertrophy and diastolic dysfunction. Diagnosis of LV hypertrophy is made when the interventricular septum (IVS) thickness and/or LV free wall (LVFW) thickness at the end diastole are >5.5 mm 3 or >6 mm 4 on the right parasternal long- or short-axis view on echocardiography in the absence of pressure overload5,6 or systemic diseases known to cause LV hypertrophy.7,8 Therefore, morphological assessment by echocardiography is very important to make a tentative diagnosis in cats with HCM.
Heart rate (HR) in cats has been shown to be greatly increased as a result of excitement in the veterinary clinic.9,10 HR reportedly influenced ventricular size, and an inverse correlation between HR and LV size was found in healthy cats. 11 Influence of alteration of HR on echocardiographic parameters, including LV wall thickness and LV chamber size, has previously been described in people and dogs.12,13 However, the degree of changes in LV wall thickness due to sudden changes of HR has not yet been assessed in cats.
We hypothesised that when HR increased, resulting in shortening of the diastolic period, LV thickness at the end diastole would increase. The purpose of this study was to evaluate the effect of sudden alterations in HR on LV wall thickness and dimensions determined by echocardiography in healthy cats.
Materials and methods
Animals
This experimental study protocol was approved by the Azabu University Animal Care and Use Committee (number 1407173).
Six experimental cats (three females and three males) aged 36 months and weighing 2.82–4.45 kg were used in this study. None of the cats were under any type of treatment. A complete clinical examination comprised of physical examination, complete blood count, blood chemistry, thoracic radiography, electrocardiography and echocardiography was performed to exclude systemic disease in all cats. None of the cats showed any abnormal findings.
The cats were kept individually in cages and fed commercially available cat food, and had free access to water.
Experimental protocol
All cats were administered intravenous propofol for induction of general anaesthesia, and maintained with isoflurane mixed with 100% oxygen. Intravenous crystalloid fluid infusion was performed at a standard maintenance rate of 2.5 ml/kg/h during anaesthesia.
Each cat was placed in right lateral recumbency, and surgical cut down was performed over the left cervical area to expose the left jugular vein. A transvenous atrial pacing lead was directly inserted into the left jugular vein, and advanced into the right atrium under fluoroscopic guidance. The lead tip was fixed in the right atrium, which was paced with an external pulse generator (External SSI Cardiac Pacemaker; Japan Medtronic).
Echocardiography and blood pressure were assessed after 2 mins of continuous atrial pacing at rates of 120, 130, 140, 150, 160, 170 and 180 ppm. The pacing rate was varied in a predetermined order in each cat and no two cats had pacing rate varied in the same order.
After recovering from anaesthesia, an antibiotic (cefazolin, 25 mg/kg) was administered via intravenous injection three times a day for 3 days following the experiment, and then cefalexin was orally administered until stitches were removed in all cats.
Echocardiographic examination
All echocardiographic images were acquired using an ultrasound unit equipped with a 7 MHz transducer (Vivid 7 Dimension; GE Medical System). Echocardiographic examination was performed by an ACVIM diplomate (YF). All echocardiographic measurements were performed by KS and YO, and directly performed on the screen freeze-frame images. The mean values of variables in five consecutive cardiac cycles were used for statistical analysis.
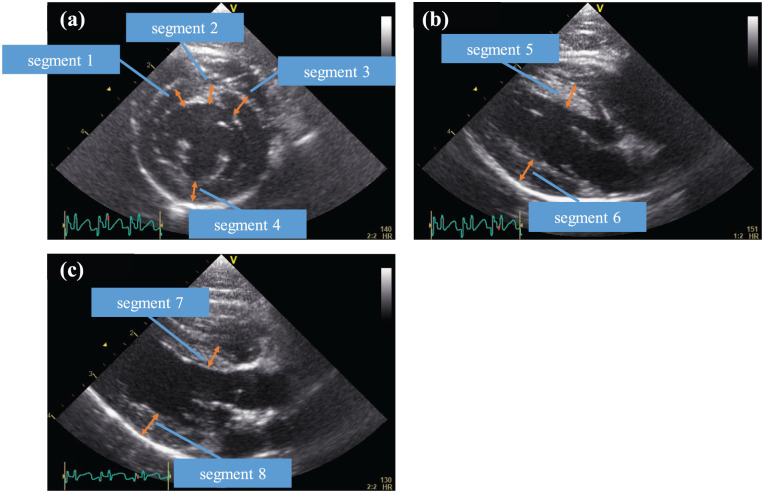
The segmental measurements were determined by referring to a previous report. 14 Interventricular septum and LVFW thickness at end diastole (IVSd and LVFWd, respectively) were measured in four segments at the right parasternal short-axis chordae tendineae level (Figure 1a), two segments at the right parasternal long axis view (Figure 1b) and two segments at the right parasternal long axis LV outflow view (Figure 1c) in each HR setting. LV end diastolic diameter (LVIDd) and LV end-systolic diameter (LVIDs) were measured at the right parasternal short-axis chordae tendineae (Figure 1a), and the LV shortening fraction (FS) was then calculated.
Figure 1.
Echocardiographic measurements of end-diastolic left ventricular (LV) wall thickness. (a) Short-axis view at the chordae tendineae level; (b) right parasternal long-axis view; (c) right parasternal long-axis LV outflow view
The wall thickness in each view was measured at end diastole, defined as either the frame immediately after closure of the mitral valve or the frame of the maximal LV diameter. 15 The thickness of the IVS was measured in all cats from the leading edge of the right ventricular side of the septum to the trailing edge of the LV side of the septum, including the endocardium but excluding right and LV papillary muscles and insertion of false tendons. The LVFWd from a right parasternal long-axis view was assessed between the mitral annulus and the posterior papillary muscle, including the endocardium but excluding the pericardium.
Systolic blood pressure
An indirect measurement of blood pressure (BP) was carefully obtained in conscious cats by use of a standardised method of the general technical protocol recommended for a BP measurement session by the American College of Veterinary Internal Medicine. 16 Systolic BP (SBP) was obtained non-invasively using Doppler sphygmomanometry (Hadeco). An inflatable cuff with appropriate size was placed on the tail. The hair was clipped before placing the probe. The cuff was manually inflated until the pulse signal was no longer audible, and then gradually deflated. SBP was determined when the Doppler signal was re-audible. Several consecutive BP measurements were undertaken during each session to obtain a stable set of five values; the mean was used for the statistical analyses.
Statistical analyses
All measurements were expressed as mean ± SD. Statistical analyses were performed using commercial computer software (SPSS Statistics version 21.0; IBM). All echocardiographic data and SBP were visually inspected and tested for normality by the Kolmogorov–Smirnov test. Correlations between HR and wall thickness, HR and LVIDd, and HR and SBP were examined by use of linear mixed model.
Intra- and inter-observer variability for each segment was assessed by calculation of coefficients of variation (CV) using the formula: CV = (SD/arithmetic mean of measurements) × 100. 17 CV were considered clinically acceptable if <10%. 18
Results
One cat was excluded from the analysis at an HR of 120 ppm because the original HR exceeded the pacing rate at 120 ppm.
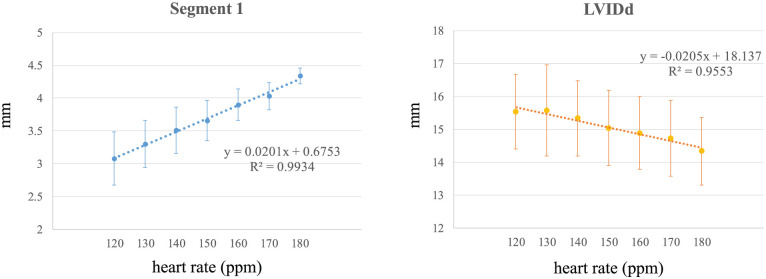
Results of each wall thickness, LVIDd, LVIDs and FS at different HRs are shown in Table 1 and Figure 2. HR and all segments of IVS, HR and LVFW (P <0.001, R2 = 0.98–0.99), HR and LVIDd (P <0.001, R2 = 0.96), HR and LVIDs (P <0.001, R2 = 0.97) and HR and FS (P <0.001, R2 = 0.89) had a linear relation. The maximum difference in single measurements between 120 ppm and 180 ppm was 2.0 mm in segment 1 in one cat, and the minimum difference was 0.7 mm in segments 3 and 4 in the other cats.
Table 1.
Results of wall thickness of each segment, LVIDd, LVIDs and FS at different HRs
| Segment | Unit | 120 ppm | 130 ppm | 140 ppm | 150 ppm | 160 ppm | 170 ppm | 180 ppm | P value | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | mm | 3.12 ± 0.32 | 3.39 ± 0.31 | 3.58 ± 0.26 | 3.79 ± 0.33 | 3.96 ± 0.21 | 4.09 ± 0.20 | 4.24 ± 0.22 | <0.001 | 0.99 |
| 2 | mm | 3.13 ± 0.33 | 3.41 ± 0.32 | 3.56 ± 0.24 | 3.79 ± 0.31 | 3.96 ± 0.25 | 4.14 ± 0.28 | 4.28 ± 0.34 | <0.001 | 0.99 |
| 3 | mm | 3.17 ± 0.33 | 3.42 ± 0.29 | 3.60 ± 0.26 | 3.81 ± 0.32 | 3.96 ± 0.24 | 4.11 ± 0.27 | 4.24 ± 0.33 | <0.001 | 0.99 |
| 4 | mm | 3.19 ± 0.31 | 3.44 ± 0.30 | 3.62 ± 0.29 | 3.84 ± 0.32 | 4.03 ± 0.23 | 4.12 ± 0.29 | 4.28 ± 0.39 | <0.001 | 0.99 |
| 5 | mm | 3.08 ± 0.40 | 3.30 ± 0.35 | 3.51 ± 0.35 | 3.66 ± 0.31 | 3.90 ± 0.24 | 4.03 ± 0.21 | 4.34 ± 0.12 | <0.001 | 0.99 |
| 6 | mm | 3.13 ± 0.39 | 3.34 ± 0.37 | 3.72 ± 0.04 | 3.90 ± 0.10 | 4.09 ± 0.18 | 4.23 ± 0.25 | 4.42 ± 0.27 | <0.001 | 0.99 |
| 7 | mm | 3.23 ± 0.33 | 3.39 ± 0.36 | 3.58 ± 0.31 | 3.78 ± 0.27 | 3.94 ± 0.21 | 4.09 ± 0.16 | 4.30 ± 0.22 | <0.001 | 0.99 |
| 8 | mm | 3.23 ± 0.34 | 3.36 ± 0.36 | 3.57 ± 0.30 | 3.77 ± 0.24 | 3.91 ± 0.17 | 4.07 ± 0.17 | 4.23 ± 0.16 | <0.001 | 0.99 |
| LVIDd | mm | 15.54 ± 1.38 | 15.58 ± 1.38 | 15.34 ± 1.14 | 15.04 ± 1.14 | 14.89 ± 1.10 | 14.73 ± 1.16 | 14.34 ± 1.02 | <0.001 | 0.96 |
| LVIDs | mm | 11.16 ± 1.13 | 10.68 ± 1.00 | 10.47 ± 1.00 | 10.23 ± 0.78 | 10.08 ± 0.69 | 9.50 ± 0.58 | 9.03 ± 0.67 | <0.001 | 0.97 |
| FS | % | 28.23 ± 4.23 | 31.18 ± 6.02 | 31.65 ± 5.79 | 31.82 ± 4.24 | 32.18 ± 4.29 | 35.14 ± 6.02 | 36.92 ± 3.73 | <0.001 | 0.89 |
HR = heart rate; LVIDd = left ventricular end-diastolic diameter; LVIDs = left ventricular end-systolic diameter; FS = left ventricular shortening fraction
Figure 2.
Results of segment 1 and left ventricular end-diastolic diameter (LVIDd) at different heart rates
SBP did not vary significantly at different HRs (Table 2).
Table 2.
Systolic blood pressure (SBP) at different heart rates (HRs)
| HR | 120 ppm | 130 ppm | 140 ppm | 150 ppm | 160 ppm | 170 ppm | 180 ppm | P value | R2 |
|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | 87.7 ± 19.8 | 91.3 ± 20.2 | 92.9 ± 17.9 | 82.3 ± 16.1 | 87.8 ± 20.7 | 87.4 ± 13.8 | 82.9 ± 13.6 | >0.05 | 0.29 |
The intra-observer CVs were clinically insignificant in all wall thickness measurements (range 3.8–5.8%) and LVIDd (range 2.5–4.5%). The inter-observer CVs were also considered clinically insignificant in all wall thickness measurements (range 3.1–8.2%) and LVIDd (range 2.6–5.5%).
Discussion
This is the first report to investigate the influence of alterations in HR on LV dimensions in healthy cats. Echocardiography is one of the most important examinations used to diagnose HCM in cats. Wall thickness has been used in diagnosis of myocardial hypertrophy, the severity of HCM 19 and index of prognosis. 20 In this study, IVSd and LVFWd were strongly correlated with HR. Although wall thickness was not 6.0 mm or more in all cats, the maximum difference between 120 ppm and 180 ppm was 2.0 mm. Thus, wall thickness could have been overestimated or underestimated by alterations in HR. The echocardiographic changes may be sufficient to confound the diagnosis or to assess the severity of HCM in some cats.
The correlations between HR and wall thickness or LVIDd have been described in people and dogs.12,13 There was a significant positive correlation between LVIDs and HR, and FS and HR in this study. The Bowditch staircase effect could explain this result. Myocardial contractility increases with an increase in HR, and a decrease in HR shows a negative staircase effect. 21 Mulieri et al reported that the Bowditch staircase effect was not observed in patients with impaired myocardial function due to mitral regurgitation. 22 Although the pathophysiology of HCM is different from that of mitral regurgitation, the influence of increased or decreased HR may not be the same in the diseased heart. 23
Atrial pacing was used in this study to bring about changes in HR. This technique permitted atrial contraction, atrial–ventricular synchrony and normal ventricular activation to be maintained. Previous studies in people and dogs have demonstrated the validity of atrial pacing as a method of altering HR to examine the effects on echocardiographic measurements.12,13
There are several limitations to the present study. First, the number of cats available was limited. Second, cats with normal cardiac function were used. Heart disease with decreased cardiac function might show different results from our study. Further study is required on the effect of HR on wall thickness and chamber dimension in cats with cardiomyopathy. Third, this study was performed under general anaesthesia. Propofol reportedly does not alter the diastolic function in cats with HCM, 24 and the cardiac index was maintained in cats under anaesthesia maintained with isoflurane. 25 However, because of the study design, the effects of anaesthesia could not be completely eliminated.
Conclusions
LV wall thickness and dimensions were significantly influenced by alterations in HR. The changes were of sufficient magnitude to potentially confound the diagnosis of HCM in cats.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 2 July 2016
References
- 1. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 3. Godiksen MT, Granstrom S, Koch J, et al. Hypertrophic cardiomyopathy in young Maine Coon cats caused by the p.A31P cMyBP-C mutation – the clinical significance of having the mutation. Acta Vet Scand 2011; 53: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995; 92: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 5. Hori Y, Uechi M, Indou A. Effects of changes in loading conditions and heart rate on the myocardial performance index in cats. Am J Vet Res 2007; 68: 1183–1187. [DOI] [PubMed] [Google Scholar]
- 6. Campbell FE, Kittleson MD. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med 2007; 21: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 7. Abbott JA. Feline hypertrophic cardiomyopathy: an update. Vet Clin North Am Small Anim Pract 2010; 40: 685–700. [DOI] [PubMed] [Google Scholar]
- 8. Fox PR. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J Vet Cardiol 2003; 5: 39–45. [DOI] [PubMed] [Google Scholar]
- 9. Quimby JM, Smith ML, Lunn KF. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J Feline Med Surg 2011; 13: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbott JA. Heart rate and heart rate variability of healthy cats in home and hospital environments. J Feline Med Surg 2005; 7: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobs G, Knight DH. M-mode echocardiographic measurements in non-anesthetized healthy cats: effects of body weight, heart rate, and other variables. Am J Vet Res 1985; 46: 1705–1711. [PubMed] [Google Scholar]
- 12. Jacobs G, Mahjoob K. Influence of alterations in heart rate on echocardiographic measurements in the dog. Am J Vet Res 1988; 49: 548–552. [PubMed] [Google Scholar]
- 13. DeMaria AN, Neumann A, Schubart PJ, et al. Systematic correlation of cardiac chamber size and ventricular performance determined with echocardiography and alterations in heart rate in normal persons. Am J Cardiol 1979; 43: 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Marz I, Wilkie LJ, Harrington N, et al. Familial cardiomyopathy in Norwegian Forest Cats. J Feline Med Surg 2015; 17: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010; 12: 171–182. [DOI] [PubMed] [Google Scholar]
- 16. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 17. Chetboul V, Athanassiadis N, Concordet D, et al. Observer-dependent variability of quantitative clinical endpoints: the example of canine echocardiography. J Vet Pharmacol Ther 2004; 27: 49–56. [DOI] [PubMed] [Google Scholar]
- 18. Granstrom S, Pipper CB, Mogelvang R, et al. Effect of sample volume size and sampling method on feline longitudinal myocardial velocity profiles from color tissue Doppler imaging. J Vet Cardiol 2012; 14: 479–488. [DOI] [PubMed] [Google Scholar]
- 19. Wess G, Sarkar R, Hartmann K. Assessment of left ventricular systolic function by strain imaging echocardiography in various stages of feline hypertrophic cardiomyopathy. J Vet Intern Med 2010; 24: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 20. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 21. Holubarsch C, Ruf T, Goldstein DJ, et al. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation 1996; 94: 683–689. [DOI] [PubMed] [Google Scholar]
- 22. Mulieri LA, Leavitt BJ, Martin BJ, et al. Myocardial force-frequency defect in mitral regurgitation heart failure is reversed by forskolin. Circulation 1993; 88: 2700–2704. [DOI] [PubMed] [Google Scholar]
- 23. Sugimoto K, Fujii Y, Sunahara H, et al. Assessment of left ventricular longitudinal function in cats with subclinical hypertrophic cardiomyopathy using tissue Doppler imaging and speckle tracking echocardiography. J Vet Med Sci 2015; 77: 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacDonald KA, Kittleson MD, Garcia-Nolen T, et al. Tissue Doppler imaging and gradient echo cardiac magnetic resonance imaging in normal cats and cats with hypertrophic cardiomyopathy. J Vet Intern Med 2006; 20: 627–634. [DOI] [PubMed] [Google Scholar]
- 25. Hodgson DS, Dunlop CI, Chapman PL, et al. Cardiopulmonary effects of anesthesia induced and maintained with isoflurane in cats. Am J Vet Res 1998; 59: 182–185. [PubMed] [Google Scholar]