Abstract
Objectives
The objective was to describe ultrasonographic characteristics of cats with stable chronic kidney disease (CKD) and determine if these were significantly different from cats with pyelonephritis (Pyelo) and ureteral obstruction (UO), to aid in clinical assessment during uremic crisis.
Methods
Sixty-six cats with stable CKD were prospectively enrolled, as well as normal control cats (n = 10), cats with a clinical diagnosis of Pyelo (n = 13) and cats with UO confirmed by surgical resolution (n = 11). Renal ultrasound was performed and routine still images and cine loops were obtained. Analysis included degree of pelvic dilation, and presence and degree of ureteral dilation. Measurements were compared between groups using non-parametric one-way ANOVA with Dunn’s post-hoc analysis.
Results
In total, 66.6% of CKD cats had measurable renal pelvic dilation compared with 30.0% of normal cats, 84.6% of Pyelo cats and 100% of UO cats. There was no statistically significant difference in renal pelvic widths between CKD cats and normal cats, or CKD cats and Pyelo cats. On almost all measurement categories, UO cats had significantly greater renal pelvic widths compared with CKD cats and normal cats (P <0.05) but not Pyelo cats. Six percent of stable CKD cats had measurable proximal ureteral dilation on one or both sides vs 46.2% of Pyelo cats and 81.8% of UO cats. There was no statistically significant difference in proximal ureteral width between normal and CKD cats, or between Pyelo and UO cats. There was a statistically significant difference in proximal ureteral width between CKD and Pyelo cats, CKD and UO cats, normal and UO cats, and normal and Pyelo cats.
Conclusions and relevance
No significant difference in renal pelvic widths between CKD cats and Pyelo cats was seen. These data suggest CKD cats should have a baseline ultrasonography performed so that abnormalities documented during a uremic crisis can be better interpreted.
Introduction
Renal ultrasound is commonly used to assess cats with azotemia, particularly during uremic crisis events. Abnormalities may include dilation of the renal pelvis, termed pyelectasia or hydronephrosis depending on the degree (with hydronephrosis, dilation includes the diverticula, as well as the renal pelvis) and dilation of the ureter. 1 Presence of such dilation may be an indication of conditions such as obstruction of the urinary tract distal to the kidney (ureteroliths, ureteral stricture, ureteritis, masses involving the ureteral opening or impinging upon the ureter, urethral obstruction) or pyelonephritis.1–5 However, dilation can also occur as a result of changes associated with chronic kidney disease, as well as with fluid therapy.1,2 Therefore, interpreting the clinical significance of pelvic dilation can often be challenging for the practitioner. The purpose of this study was to determine ultrasonographic characteristics of the renal pelvis and proximal ureter of cats with stable chronic kidney disease (CKD) and determine if these were significantly different enough from cats with pyelonephritis and ureteral obstruction (UO), to aid in clinical diagnosis of the latter conditions in times of uremic crisis.
Materials and methods
Cats
Sixty-six cats with clinically stable CKD were enrolled (‘CKD cats’), as well as normal control cats (n = 10; ‘normal cats’), cats with a clinical diagnosis of pyelonephritis (n = 13; ‘Pyelo cats’) and cats with UO confirmed by surgical resolution (n = 11; ‘UO cats’). Stable CKD cats were defined as those with previously diagnosed and staged CKD that presented to Colorado State University medicine service for screening to be included in clinical research trials. CKD staging was determined according to International Renal Interest Society (IRIS) guidelines and is based on serum creatinine assessed on at least two occasions in a stable, hydrated patient: IRIS CKD stage 1 cats have creatinine <1.6 mg/dl with other evidence of renal abnormality such as urine specific gravity <1.035 or abnormal kidneys on palpation or imaging; IRIS CKD stage 2 cats have creatinine 1.6–2.8 mg/dl; IRIS CKD stage 3 cats have creatinine 2.9–5.0 mg/dl; and IRIS CKD stage 4 cats have creatinine >5 mg/dl. Each CKD patient received a full diagnostic work-up, including physical examination, complete blood count, serum biochemistry, urinalysis, urine culture, urine protein:creatinine ratio, total thyroxine, blood pressure and abdominal ultrasound. Exclusion criteria for this group included urinary tract infection (UTI), ureteroliths, uncontrolled systemic disease, elevation in serum creatinine from previous baseline or other indication that the patient was not clinically stable. Two cats were excluded after ultrasound for renal masses.
Cats with a clinical diagnosis of pyelonephritis were defined as those undergoing diagnostics and treatment for uremic crisis (elevation in serum creatinine from previous baseline) with bacteriuria on urinalysis collected via cystocentesis and positive urine culture, or an active sediment suspicious of UTI on urinalysis collected via cystocentesis. Concurrent ureteroliths was an exclusion criteria for this group. These cats also had to have two or more history and physical examination parameters consistent with pyelonephritis, including anorexia or inappetence, lethargy, vomiting, periuria, hematuria, weight loss, or renal or abdominal pain. Presence of pyelectasia on ultrasound was not used as an inclusion criterion. Final determination of clinical diagnosis of pyelonephritis was made after response to therapy was assessed and repeat ultrasound was performed to confirm improvement of dilation of pelvis and/or ureters. Fifty-seven cats were initially evaluated for this category and, upon review, only 13 cases could be included with a confident clinical diagnosis of pyelonephritis.
Cats with UO were retrospectively selected for comparative purposes as those cases where UO was surgically managed and follow-up demonstrated resolution of clinical signs, elevation of creatinine from baseline and ultrasonographic abnormalities. Concurrent UTI (as determined by urine culture) was an exclusion criterion for this group. Normal cats prospectively enrolled for the basis of comparison were defined as young cats <7 years, with no historical or clinical abnormalities and normal complete blood count, chemistry and urinalysis. Owners of cats in the normal, Pyelo and CKD groups reviewed and signed consent forms pertinent to the Institutional Animal Care and Use Committee-approved study they were being screened for prior to participation. Cats in the UO group had ultrasound examination performed as part of routine clinical assessment.
Ultrasonography
Ultrasonography was performed on all 100 cats. All cats received an abdominal ultrasound examination by a board-certified radiologist (EKR) or radiology resident. The cat’s ventral abdomen was clipped from costal arch to caudal abdomen/pubic region and then positioned in dorsal recumbency for examination. Cats were not sedated, and gentle manual restraint was utilized for the ultrasound examination. Images were acquired on a Siemens Acuson Anteres or Siemens Acuson Sonoline ultrasound machine utilizing a 9–4 MHz linear transducer. Cine loops and still images of both kidneys were obtained in both sagittal and transverse planes. Measurements were performed by a board-certified radiologist (EKR). Cine loops and still images were evaluated for the maximal degree of renal pelvic dilation in each view. The following measurements were obtained.
Sagittal renal pelvic width
In the sagittal (Sag) plane, a dilated renal pelvis was identified as an elongated area of anechoic dilation bordered by thin hyperechoic walls of the central renal sinus. The calipers were placed at the internal surface of each wall.
Transverse renal pelvic width
In the transverse (Trans) plane, a dilated pelvis was identified as a crescent-shaped anechoic structure around the renal crest. Calipers were placed at the internal surface of the margins of the pelvis and care was taken not to include the proximal ureter. Non-dilated pelves were attributed a measurement of 0 mm.
Sag renal pelvic width
Average Sag renal pelvic width was determined as the average of left and right renal pelvic width measurements in the Sag plane.
Average Trans renal pelvic width
Average Trans renal pelvic width was determined as the average of left and right renal pelvic width measurements in the Trans plane.
Greatest Sag renal pelvic width
Greatest Sag renal pelvic width was determined as the larger of the two (left or right) renal pelvic width measurements in the Sag plane.
Greatest Trans renal pelvic width
Greatest Trans renal pelvic width was determined as the larger of the two (left or right) renal pelvic width measurements in the Trans plane.
Proximal ureteral width
Proximal ureteral width was determined by measuring the proximal ureter if it was visible and recording the largest diameter from both Sag and Trans plane. Non-visible ureters were attributed a measurement of 0 mm. Average proximal ureteral width was determined as the average of left and right proximal ureteral width measurements. Greatest proximal ureteral width was determined as the larger of the two (left or right) proximal ureteral width measurements.
Statistical analysis
The degree of renal pelvic dilation and ureteral dilation were compared for each plane and side (right Sag renal pelvic width, left Sag renal pelvic width, right Trans renal pelvic width, left Trans renal pelvic width, average Sag renal pelvic width, average Trans renal pelvic width, greatest Sag renal pelvic width, greatest Trans renal pelvic width, average proximal ureteral width, greatest proximal ureteral width) between the four groups (Normal, CKD, Pyelo, UO) using a non-parametric one-way ANOVA (Kruskal–Wallis) with Dunn’s post-hoc analysis in Prism software (Prism 5; GraphPad). Fisher’s exact test was used to analyze correlation of ureteral dilation and stones with each study group, as well as correlation of subcutaneous (SC) fluids with pyelectasia within the CKD group. Data were assessed for normality using the D’Agostino and Pearson normality test and were not found to be normally distributed; hence, non-parametric tests were used for analysis. For all analyses, a P value <0.05 was considered to be statistically significant.
Results
Cats
The 10 cats (three spayed females, seven neutered males) included in the normal group included nine domestic shorthairs and one Siamese. The median age was 2 years (range 1–7 years). The 66 cats (28 spayed females, 38 neutered males) included in the CKD group included 42 domestic shorthairs, 11 domestic longhairs, nine Siamese, and one each of Tonkinese, Burmese, Russian Blue and Maine Coon. The median age was 14 years (range 5–18 years). CKD cats consisted of IRIS CKD stage 1 (n = 2), IRIS CKD stage 2 (n = 31), IRIS CKD stage 3 (n = 27) and IRIS CKD stage 4 (n = 6). The 13 cats (six spayed females, seven neutered males) in the Pyelo group included eight domestic shorthairs, two domestic longhairs, and one each of Himalayan, Ragdoll and Bengal. The median age was 13 years (range 4–16 years). The 11 (five spayed females, six neutered males) cats in the UO group included nine domestic shorthairs, one domestic longhair and one Siamese. The median age was 8 years (range 4–16 years).
Renal pelvic ultrasonography
Sixty-six percent of cats with stable CKD had measurable renal pelvic dilation vs 30.0% of normal cats, 84.6% of Pyelo cats and 100% of UO cats. Sag and Trans renal pelvic width measurements for both kidneys, average of left and right pelvic width measurements and greatest of left or right pelvic width measurements are presented in Table 1. Renal pelvic width measurements for left and right Trans and Sag views are presented in Figure 1 (a,b). Average renal pelvic widths are presented in Figure 2 (a,b). Greatest renal pelvic width measurements are presented in Figure 3 (a,b).
Table 1.
Measurements of renal pelvic dilation and proximal ureteral dilation in the four study groups, including pelvic and ureteral width measurements for both kidneys, average of left and right renal pelvic and ureteral width, and greatest pelvic and ureteral width of two (left or right) kidney measurements
| Normal (n = 10) | CKD (n = 66) | Pyelo (n = 13) | UO (n = 11) | ||
|---|---|---|---|---|---|
| Sagittal renal pelvic diameter (mm) | |||||
| Left kidney ‡ | Average ± SD | 0 | 1.3 ± 2.5 | 1.9 ± 2.7 | 6.8 ± 8.1 |
| Median (range) | 0 (0–0) | 0 (0–11.5) | 0.8 (0–8.3) | 3.5 (0–24.7) | |
| Right kidney ‡ ¶ ∞ | Average ± SD | 0 | 0.88 ± 1.8 | 1.9 ± 2.4 | 8.9 ± 6.3 |
| Median (range) | 0 (0–0) | 0 (0–10.3) | 0 (0–7.1) | 6.0 (1.6–17.9) | |
| Average † ‡ ¶ ∞ | Average ± SD | 0 | 1.1 ± 1.9 | 1.9 ± 2.3 | 7.8 ± 5.0 |
| Median (range) | 0 (0–0) | 0.25 (0–8.3) | 1.1 (0–6.9) | 7.7 (2.0–15.6) | |
| Greatest † ‡ ¶ | Average ± SD | 0 | 1.6 ± 2.7 | 2.6 ± 2.8 | 11.3 ± 7.2 |
| Median (range) | 0 (0–0) | 0.5 (0–11.5) | 2.1 (0–8.3) | 10.7 (2.8–24.7) | |
| Transverse renal pelvic diameter (mm) | |||||
| Left kidney † ‡ | Average ± SD | 0.12 ± 0.26 | 1.4 ± 2.2 | 2.7 ± 3.1 | 5.8 ± 6.1 |
| Median (range) | 0 (0–0.7) | 0.85 (0–12.5) | 1.5 (0–10.1) | 4.1 (0–19.3) | |
| Right kidney † ‡ ¶ | Average ± SD | 0.12 ± 0.25 | 1.0 ± 1.8 | 2.4 ± 2.7 | 8.6 ± 5.5 |
| Median (range) | 0 (0–0.6) | 0 (0–12) | 2.5 (0–9.1) | 9.7 (1.5–17.2) | |
| Average † ‡ ¶ | Average ± SD | 0.12 ± 0.2 | 1.2 ± 1.7 | 2.6 ± 2.6 | 7.2 ± 3.6 |
| Median (range) | 0 (0–0.55) | 0.65 (0–7.5) | 2.0 (0–9.6) | 7.2 (2.2–13.5) | |
| Greatest † ‡ ¶ | Average ± SD | 0.19 ± 0.3 | 1.7 ± 2.6 | 3.2 ± 3.1 | 10.5 ± 5.5 |
| Median (range) | 0 (0–0.7) | 1.0 (0–12.5) | 2.5 (0–10.1) | 10.4 (3.1–19.3) | |
| Proximal ureteral diameter (mm) | |||||
| Left kidney ‡ § ¶ | Average ± SD | 0 | 0.11 ± 0.67 | 1.3 ± 2.0 | 2.6 ± 4.2 |
| Median (range) | 0 (0–0) | 0 (0–5.1) | 0 (0–4.4) | 0 (0–14) | |
| Right kidney ‡ § ¶ | Average ± SD | 0 | 0.13 ± 0.61 | 1.2 ± 1.8 | 3.8 ± 4.0 |
| Median (range) | 0 (0–0) | 0 (0–3.7) | 0 (0–5.3) | 3.2 (0–14) | |
| Average † ‡ § ¶ | Average ± SD | 0 | 0.12 ± 0.49 | 1.2 ± 1.7 | 3.2 ± 4.8 |
| Median (range) | 0 (0–0) | 0 (0–2.6) | 0 (0–4.8) | 2.7 (0–14) | |
| Greatest † ‡ § ¶ | Average ± SD | 0 | 0.2 ± 0.9 | 1.7 ± 2.1 | 4.2 ± 3.8 |
| Median (range) | 0 (0–0) | 0 (0–5.1) | 0 (0–5.3) | 3.7 (0–14) |
Significant difference between normal and CKD
Significant difference between normal and Pyelo
Significant difference between normal and UO
Significant difference between CKD and Pyelo
Significant difference between CKD and UO
Significant difference between Pyelo and UO
CKD = chronic kidney disease; Pyelo = pyelonephritis; UO = ureteral obstruction
Figure 1.
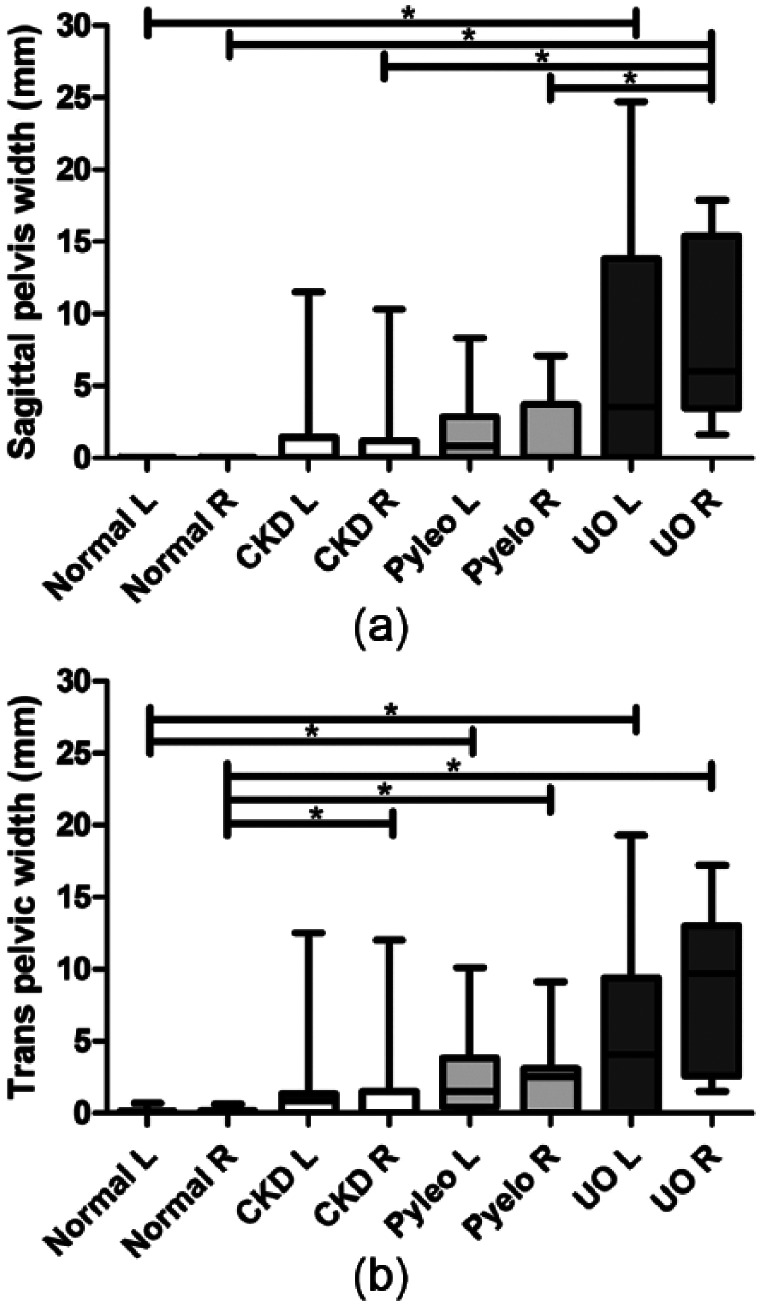
Renal pelvic width measurements for (a) sagittal left (L) and right (R) and (b) transverse left and right planes are presented as box and whisker plots. Statistically significant differences between normal, chronic kidney disease (CKD), pyelonephritis (Pyelo) and ureteral obstruction (UO) groups are denoted by an asterisk (P <0.05)
Figure 2.
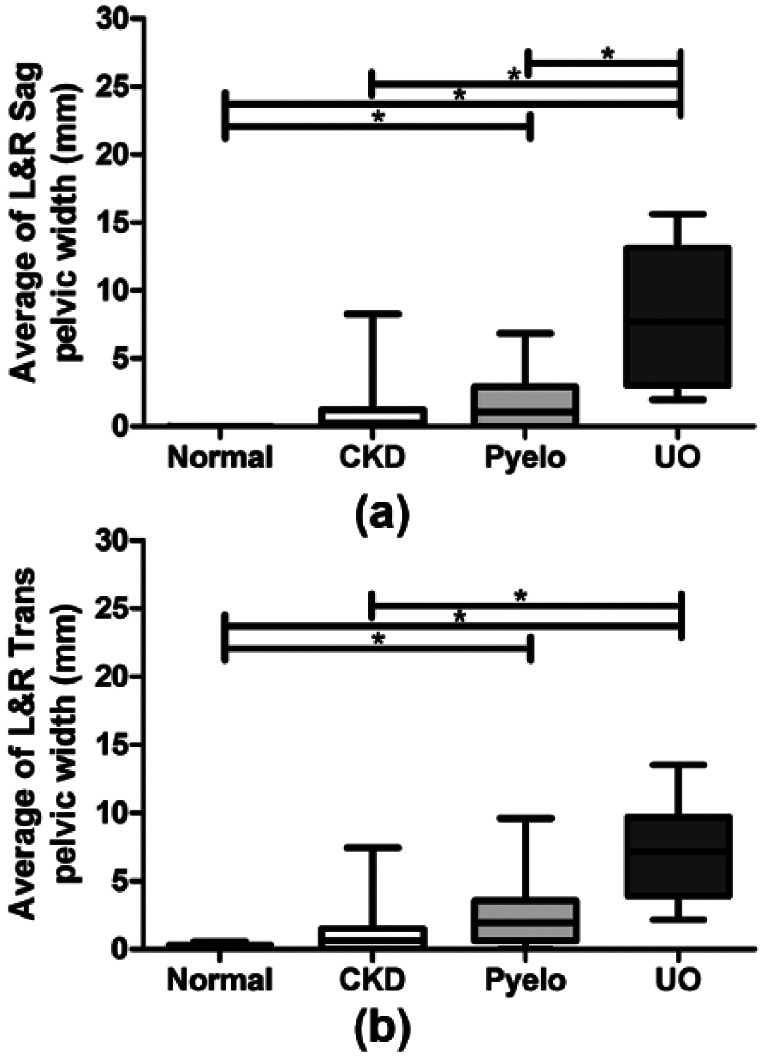
Average of left and right (L&R) renal pelvic width measurements in (a) sagittal and (b) transverse planes are presented as box and whisker plots. Statistically significant differences between normal, chronic kidney disease (CKD), pyelonephritis (Pyelo) and ureteral obstruction (UO) groups are denoted by an asterisk (P <0.05)
Figure 3.
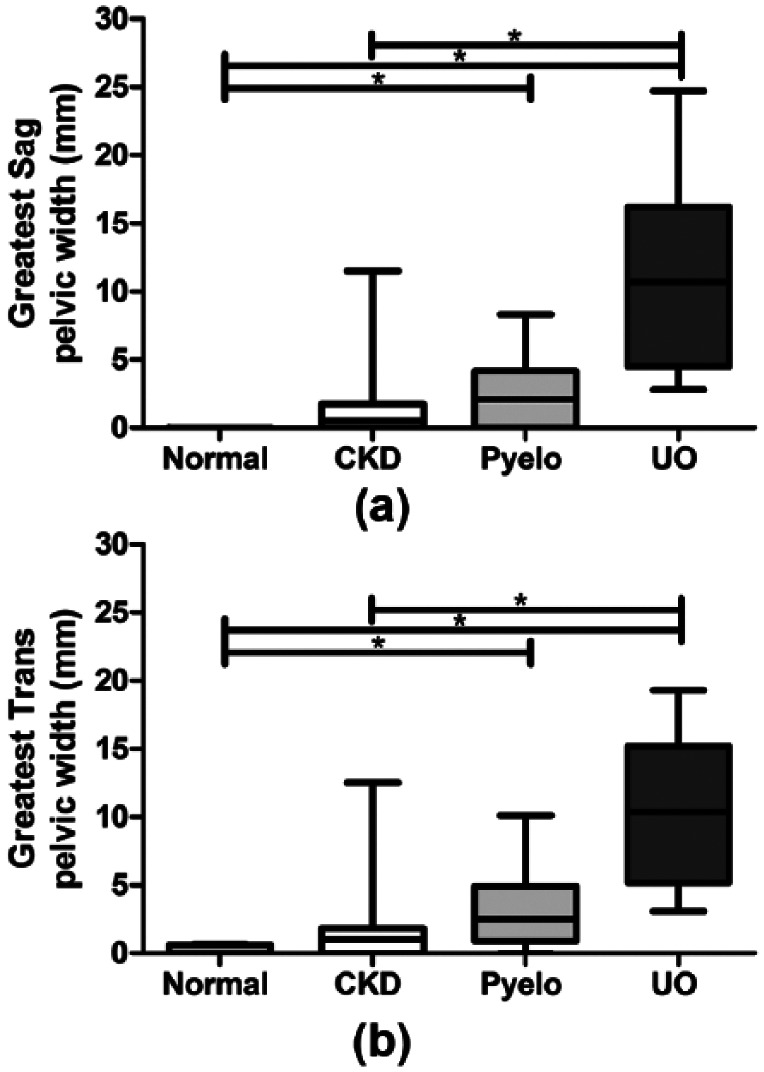
Greatest of two (left and right) renal pelvic width measurements in (a) sagittal (Sag) and (b) transverse (Trans) planes are presented as box and whisker plots. Statistically significant differences between normal, chronic kidney disease (CKD), pyelonephritis (Pyelo) and ureteral obstruction (UO) groups are denoted by an asterisk (P <0.05)
Regardless of the category of measurement used (plane, side, average or greatest width), there was no statistically significant difference in renal pelvic widths between CKD cats and normal cats or CKD cats and Pyelo cats. For the majority of measurement categories, Pyelo cats had significantly greater renal pelvic widths compared with normal cats. On almost all measurement categories, UO cats had significantly greater renal pelvic widths compared with CKD cats (P <0.05) and normal cats (P <0.05) but not Pyelo cats. However, UO cats did have significantly greater renal pelvic widths compared with Pyelo cats on the right, average and greatest Sag measurements (P <0.05).
There was no correlation between the degree of renal pelvic dilation and serum creatinine or IRIS stage within the CKD cat group. There was no significant difference in the percentage of CKD cats with renal pelvic dilation between IRIS stages (stage 1: 50%; stage 2: 65%; stage 3: 70%; stage 4: 66%). Distribution of renal pelvic dilation (unilateral vs bilateral, symmetric vs asymmetric, side) is presented in Table 2. Of CKD cats that had renal pelvic dilation, the cats were significantly more likely to have bilateral renal pelvic dilation than unilateral (P = 0.002), with no significant difference in symmetry. Of CKD cats that had unilateral renal pelvic dilation, left-sided dilation was significantly more common (P = 0.007).
Table 2.
Distribution of renal pelvic dilation (unilateral vs bilateral, symmetric vs asymmetric, side)
| No renal pelvic dilation | Bilateral pelvic dilation | Unilateral pelvic dilation | |
|---|---|---|---|
| Normal cats (n = 10) | 7/10 (70%) | 1/10 (10%): symmetric 1/1 (100%); asymmetric 0/1 (0%) |
2/10 (20%): left 1/2 (50%); right 1/2 (50%) |
| Chronic kidney disease cats (n = 66) | 23/66 (35%) | 29/66 (44%): symmetric 16/29 (55%); asymmetric 13/29 (45%) |
14/66 (21%): left 11/14 (79%); right 3/14 (21%) |
| Pyelonephritis cats
(n = 13) |
2/13 (15%) | 8/13 (62%): symmetric 5/8 (63%); asymmetric 3/8 (37%) |
3/13 (23%): left 2/3 (67%); right 1/3 (33%) |
| Ureteral obstruction cats (n = 11) | 0/11 (0%) | 8/11 (73%): symmetric 3/8 (37%); asymmetric 5/8 (63%) |
3/11 (27%): left 0/3 (0%); right 3/3 (100%) |
Significance of distribution, symmetry and side could not be meaningfully evaluated in Pyelo and UO groups, owing to small group number. It was noted that renal pelvic dilation of >13 mm was invariably attributable to UO.
Proximal ureteral ultrasonography
Six percent of stable CKD cats had measurable proximal ureteral dilation on one or both sides vs 46% of Pyelo cats and 82% of UO cats. CKD cats were significantly less likely to have proximal ureteral dilation compared with Pyelo cats (P = 0.0009) and UO cats (P <0.0001) No ureteral dilation was found in normal cats. Average ± SD, and median and range of proximal ureteral dilation are presented in Table 1. On all ureteral measurement categories there was a statistically significant difference in proximal ureteral width between CKD and Pyelo cats, CKD and UO cats, and normal and UO cats. Regardless of the category of measurement used (side, average or greater width), there was no statistically significant difference in proximal ureteral width between normal and CKD cats and Pyelo and UO cats. When average or greater proximal ureteral width was used there was also a statistically significant difference between normal and Pyelo cats. Distribution of proximal ureteral dilation (unilateral vs bilateral, symmetric vs asymmetric, side) is presented in Table 3. Significance of distribution of ureteral dilation could not be meaningfully evaluated in Pyelo and UO groups, owing to small number of affected cats. Correlation between ureteral dilation and creatinine or IRIS stage in CKD cats could not be meaningfully evaluated due to small number of affected cats.
Table 3.
Distribution of proximal ureteral dilation (unilateral vs bilateral, symmetric vs asymmetric, side)
| No ureteral dilation | Bilateral ureteral dilation | Unilateral ureteral dilation | |
|---|---|---|---|
| Normal cats (n = 10) | 10/10 (100%) | NA | NA |
| Chronic kidney disease cats (n = 66) | 62/66 (94%) | 1/4 (25%): symmetric 0/1 (0%); asymmetric 1/1 (100%) |
3/4 (75%): left 1/3 (33%); right 2/3 (67%) |
| Pyelonephritis cats (n = 13) | 6/13 (46%) | 3/6 (50%): symmetric 1/3 (33%); asymmetric 2/3 (67%) |
3/6 (50%): left 1/3 (33%); right 2/3 (67%) |
| Ureteral obstruction cats (n = 11) | 2/11 (18%) | 4/9 (44%): symmetric 1/4 (25%); asymmetric 3/4 (75%) |
5/9 (56%): left 1/5 (20%); right 4/5 (80%) |
NA = not applicable
Association with fluid administration
No cats in the normal or CKD groups were receiving intravenous (IV) fluids at the time of ultrasound. Eleven (16.6%) of the CKD cats were receiving SC fluid therapy at the time of ultrasound. There was no correlation between administration of SC fluids and presence of renal pelvic dilation on ultrasound in CKD cats. Four cats in the Pyelo group were receiving IV fluid therapy, two cats were receiving SC fluid therapy and seven cats were not receiving fluids at the time of ultrasound. Four cats in the UO group were receiving IV fluids therapy, two cats were receiving SC fluid therapy and seven cats were not receiving fluids at the time of ultrasound. Numbers in these subgroups were inadequate for meaningful statistical comparison.
Discussion
The purpose of this study was to describe ultrasonographic characteristics of the renal pelvis and proximal ureter of cats with stable CKD and determine if these were significantly different enough from Pyelo cats and UO cats to aid in clinical assessment during uremic crisis. When renal ultrasound was performed in these groups a significant number (66.6%) of CKD cats had measurable renal pelvic dilation, and pelvic width measurements were not significantly different between CKD cats and Pyelo cats, reflecting a substantial amount of overlap in the degree of renal pelvic dilation between CKD and Pyelo cats. The degree of dilation documented in some CKD cats was surprising, particularly given the apparent lack of clinical effect on the patient. As an example, one stable IRIS stage 2 CKD cat with Sag renal pelvic dilation of 12.5 mm had a creatinine of 2.1 mg/dl and experienced no progression of disease and had unchanged ultrasound findings for 4 years after initial ultrasound evaluation (Figure 4). Unfortunately, long-term follow-up was not available for the majority of cats assessed in the study so correlation between ultrasound findings and clinical outcome could not be explored more objectively.
Figure 4.
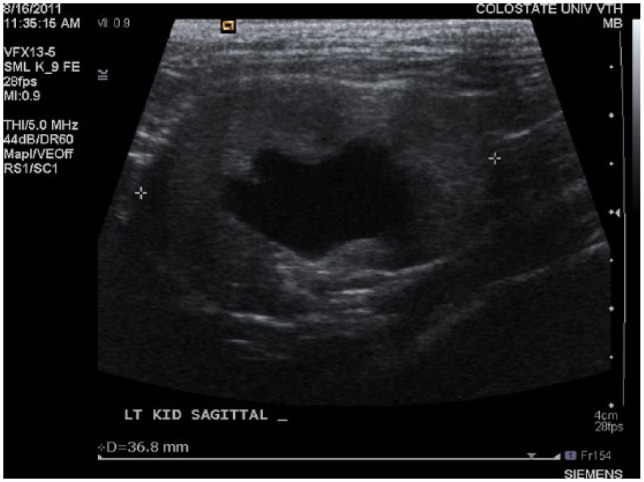
Cat with stable International Renal Interest Society stage 2 chronic kidney disease with a creatinine of 2.1 mg/dl, showing 12.5 mm renal pelvic dilation left transverse plane and 9.8 mm renal pelvic dilation left sagittal plane. This patient experienced no progression of disease and had unchanged ultrasound findings for 4 years after initial ultrasound evaluation
Similar to the difficulty in distinguishing CKD from Pyelo cats, there was a substantial amount of overlap in the degree of renal pelvic dilation between Pyelo and UO cats. UO cats had significantly greater renal pelvic widths compared with CKD cats on most measurements, and only had significantly greater renal pelvic widths compared with Pyelo cats on Sag plane measurements. However, similar to a previous study, in the current study renal pelvic dilation of >13 mm was invariably attributable to UO. 2
Assessment of proximal ureteral dilation added additional information as relatively few (6%) stable CKD cats had measurable proximal ureteral dilation on one or both sides compared vs 46.2% of Pyelo cats and 81.8% of UO cats. There was a statistically significant difference in proximal ureteral width between CKD and Pyelo cats, and CKD and UO cats. However, there was no statistically significant difference in proximal ureteral width between Pyelo and UO cats.
The distribution of renal pelvic dilation was most commonly bilateral in CKD cats, but this was also true of Pyelo and UO cats. Of the CKD cats that had unilateral renal pelvic dilation, it was more common on the left side (67% of CKD with pelvic dilation), the reason for which is unknown. There could be many potential causes of asymmetric renal pelvic dilation in CKD cats, including ureteral stricture, ureteritis, circumcaval ureters, masses and partial obstruction by non-radiopaque material such as blood clots or inflammatory debris.2,4–7 However, many of these would not be expected in a patient with stable disease. Additional studies, likely involving advanced imaging modalities such as a contrast computed tomography urogram, would be necessary to better characterize disease in this population of patients. Distribution of renal pelvic dilation could not be meaningfully assessed in Pyelo and UO cats in this study owing to the small number of cats. However, subjectively proximal ureteral dilation was more common on the right side in Pyelo and UO cats.
Administration of SC fluids did not correlate with the presence of pyelectasia in CKD cats. Pyleo and UO cats were more likely to be on IV fluid therapy, which may have influenced measurements as IV diuresis has previously been observed to have an effect on renal pelvic width. 2 However, this is representative of a typical scenario in clinical practice as a cat in a uremic crisis would likely be receiving some type of fluids at the time of ultrasound assessment. A limitation of the study is that data regarding fluid therapy was collected in a retrospective manner, and for Pyelo and UO cats details of fluid therapy management were variably available. Small sample size of the Pyelo and UO groups was also a limitation of this study and likely affected the potential for statistically significant subanalysis within these groups. However, the main focus of this study was to provide information about renal pelvic and proximal ureteral abnormalities in stable CKD cats.
The diagnosis of pyelonephritis can be clinically challenging and usually incorporates a global assessment of history and findings on physical examination, laboratory work, ultrasonographic imaging and response to therapy. As a result, for the purposes of inclusion in this study, Pyelo cats had to have qualifying features from all categories (history, physical examination, laboratory work and response to therapy) and ultrasonographic imaging results were not used as part of the assessment for inclusion as this was the parameter being studied. Therefore, few Pyelo cases qualified for enrollment. Of the cases that were included, the majority had bacteriuria (11/13 cats). However, despite the stringency of the inclusion criteria it cannot be entirely ruled out that some cats with simple urinary tract with a prerenal azotemia could have been included and/or that cats simply responded to supportive care. The difficulty of diagnosing pyelonephritis further serves to illustrate the importance of interpretation of ultrasound findings at the time of uremic crisis.
Conclusions
In this study a significant number of cats with clinically stable CKD had renal pelvic dilation that was similar in degree to Pyelo cats. Regardless of the category of measurement used (view, side, average or greater width), there was no statistically significant difference in renal pelvic widths between CKD cats and Pyelo cats. Assessment of proximal ureteral dilation appeared to be helpful as relatively few stable CKD cats had measurable proximal ureteral dilation on one or both sides compared with Pyelo and UO cats. Overall, owing to similarities between groups, these findings suggest that baseline ultrasound data in cats with CKD would greatly aid interpretation of findings during a uremic crisis. Otherwise, renal pelvic dilation could be misinterpreted as the cause of the uremic crisis (pyelonephritis or obstruction) when it may have been present for some time.
Acknowledgments
We gratefully acknowledge Dr Kim Yore, Dr Sara Wennogle and Dr Madeline Fujishiro for coordinating urine culture submission and assisting with case enrollment.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was supported by Frankie’s Fund for Feline Stem Cell Research and, in part, by a grant from the Colorado State University College Research Council.
Accepted: 6 June 2016
References
- 1. Debruyn K, Haers H, Combes A, et al. Ultrasonography of the feline kidney: technique, anatomy and changes associated with disease. J Feline Med Surg 2012; 14: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Anjou MA, Bedard A, Dunn ME. Clinical significance of renal pelvic dilatation on ultrasound in dogs and cats. Vet Radiol Ultrasound 2011; 52: 88–94. [PubMed] [Google Scholar]
- 3. Nevins JR, Mai W, Thomas E. Associations between ultrasound and clinical findings in 87 cats with urethral obstruction. Vet Radiol Ultrasound 2015; 56: 439–447. [DOI] [PubMed] [Google Scholar]
- 4. Leib MS, Allen TA, Konde LJ, et al. Bilateral hydronephrosis attributable to bilateral ureteral fibrosis in a cat. J Am Vet Med Assoc 1988; 192: 795–797. [PubMed] [Google Scholar]
- 5. Kyles AE, Hardie EM, Wooden BG, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984–2002). J Am Vet Med Assoc 2005; 226: 932–936. [DOI] [PubMed] [Google Scholar]
- 6. Belanger R, Shmon CL, Gilbert PJ, et al. Prevalence of circumcaval ureters and double caudal vena cava in cats. Am J Vet Res 2014; 75: 91–95. [DOI] [PubMed] [Google Scholar]
- 7. Steinhaus J, Berent AC, Weisse C, et al. Clinical presentation and outcome of cats with circumcaval ureters associated with a ureteral obstruction. J Vet Intern Med 2015; 29: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


