Abstract
Evidence suggests that apparently healthy cats presenting for routine evaluation should have a randomly sampled urine specific gravity (USG) >1.035. A USG <1.035 might reflect inappropriate concentrating ability warranting further investigation. We measured the USG of 1040 apparently healthy cats presenting to first opinion practice in an observational study, using either in-clinic refractometers or measurements provided by reference laboratories, and examined factors that might affect USG. In-clinic refractometers were calibrated using distilled water (specific gravity = 1.000). The USG was >1.030 in 91% of cats and >1.035 in 88% of cats; 121 adult cats (⩾6 months old) and five young cats (<6 months old) had USGs of <1.035. Of these 126 cats, a pathological cause was identified in 27 adult cats – of these, 26 were >9 years old – but no young cats. No cause was identified in 43 adult cats, and further investigation was not pursued in 51 adult cats. Factors that affected USG included age, diet type, sex, fasting status, drinking avidity, refractometer type, and the interaction between sex and diet – increasing dietary moisture content lowered USG only in female cats. Most factors minimally affected USG. The odds of having a USG <1.035 without apparent pathology included age and dietary moisture content. Drinking avidity decreased with increasing dietary moisture content. Our results show that most apparently healthy cats presenting to first-opinion practice should have a USG >1.035. Dietary management strategies to lower USG might be less effective than anticipated, and warrant monitoring of USG to determine efficacy. Older cats with USG <1.035 are more likely to have pathological causes identified, although clinicians are more likely to examine these cats for possible pathology. A lack of stringent refractometer calibration could have caused some errors in estimates of USG by some observers, but would be unlikely to alter markedly the findings.
Introduction
Urine specific gravity (USG) is used to assess renal function – the ability of tubules to concentrate and dilute urine to maintain fluid homeostasis. Randomly collected samples in healthy dogs have USG values ranging from 1.006 to >1.050, with early morning samples being slightly more concentrated than evening samples. 1 Healthy cats are considered to concentrate urine, resulting, in most cases, in a USG of >1.035 (SP Dibartola, personal communication),2–4 although reference intervals for randomly obtained urine samples vary, ranging from 1.001–1.080 to 1.020–1.040.5,6 One study, performed at a single institution, of 66 apparently healthy adult male cats found that the USG ranged from 1.023 to 1.084, with a median of 1.057, with only 8/104 measurements having a USG <1.035. 4 A more recent study, also performed at a single academic institution, found that 85% of 99 middle-aged and old cats had USGs of >1.035, but did not comment on the significance of this observation. 7 A further study by the same investigators found that 87% of 62 healthy cats had USGs of >1.035. 8 A study of 29 kittens found that, by 8 weeks of age, USG was in a range similar to that of adult cats. 9 However, no large-scale studies have examined USG in apparently healthy cats presenting to first opinion practice veterinarians, and no studies have examined relationships between USG and environmental factors in cats outside of an experimental setting. Furthermore, little information exists about variations in USG from feline urine samples collected at different times of the day. 3 Based on these observations, clinicians have anecdotally advised that a USG of <1.035 in an apparently healthy cat warrants investigation for a possible cause (SP Dibartola, personal communication).
Investigators have found that cats fed canned (high-moisture) diets consume more total water (through food and drinking) and have lower USGs than cats consuming dry diets.2,10–13 Similarly, the USG in healthy male adult cats decreased after oral water loading with 4–10 ml/kg of water. 4 Consequently, a dietary modification that could dilute urine (as assessed by a decreased USG) has been proposed as a management strategy for cats with obstructive and non-obstructive lower urinary tract disorders.12,14,15 Some authors have suggested a target USG of 1.025 in cats with lower urinary tract disorders. 15 However, no studies have systematically evaluated the effect of dietary modification on USG in cats with lower urinary tract disorders, nor demonstrated the magnitude of urine dilution that might result from such modifications.
We sought to examine USG in a large cohort of apparently healthy adult cats presenting to first opinion practice for routine annual evaluation, routine anesthetic procedures or minor dermatological problems. We also examined USG in a small subset of young cats. We examined multiple environmental factors that might be associated with changes in USG. We hypothesized that most apparently healthy adult cats should have a USG of >1.035, and that various environmental factors, such as diet, age, collection time and sex, could affect USG.
Methods
We designed a prospective cross-sectional study to examine the USG of apparently healthy cats. We recruited participants from the Veterinary Information Network (VIN) membership by a brief survey asking for clinicians to volunteer. Clinicians who volunteered were provided with information about the study objectives, and instructions about data collection and submission. A data collection form was distributed to each participant to allow easy data recording (Supplementary material). The information was then uploaded to an online database using a proprietary online data collection system (VIN).
All urine was collected either as part of a routine patient evaluation, or after informed client consent when not considered part of the routine evaluation. The method of urine collection was left to the discretion of the clinician, although cystocentesis was recommended.
Clinicians were instructed to calibrate their refractometers using distilled water at room temperature prior to obtaining a USG reading.
Cats were considered eligible for inclusion as ‘adult’ cats if they met the following criteria:
The cat was apparently healthy as far as the client was concerned, and was not receiving any chronic therapy other than routine preventative therapies (antiparasitic, etc)
The cat was presented to the clinician for a routine annual evaluation and/or vaccination, for a routine elective anesthetic procedure (eg, neutering, declawing, dental prophylaxis), for a non-medical reason (eg, boarding or grooming), or for a minor dermatological or behavioral complaint
The cat was a pet of the clinician or veterinary staff and not presented for any evaluation
The cat was >6 months of age
Cats were excluded if the cat presented for inappropriate elimination (voiding) or if it had a history of urinary tract disease. We did not exclude cats if they were <6 months of age, but this cohort was analyzed separately.
Information collected about each cat included age, sex, reason for presentation (visit reason), USG, time of urine sampling (early morning, mid- or late morning, afternoon, evening), fasting status (removal of food only, removal of food and water, no fasting), diet (canned, mostly canned, equal proportions of canned and dry, mostly dry, dry), lifestyle (indoor only, indoor/outdoor, outdoor only), owner-perceived drinking avidity (hardly ever drinks, drinks normally, drinks avidly, restricted from drinking excessively), analysis method (regular in-clinic refractometer, feline-specific refractometer, reference laboratory measurement).
If a clinician observed a USG <1.035, they were asked whether a medical or behavioral reason was identified to explain the USG, or whether they pursued a cause. Additionally, clinicians were asked if they repeated the USG at a later date to determine if the USG was persistently <1.035.
Following data evaluation, one of the investigators (MR) contacted clinicians submitting data demonstrating a USG of <1.035 but without follow-up or explanation for the USG to enquire as to whether additional evaluation could be performed. These results were then recorded where possible.
If a USG exceeded the upper limit of the refractometer, clinicians were asked to record the measurement as being ‘>X’ (where ‘X’ defined the upper limit of the refractometer). In such cases, the sample was ascribed a value of ‘X + 0.001’ for the purpose of analysis.
Statistical analysis
Data were evaluated by one of the investigators (MR) for integrity. Cats with identified pathology that could result in USGs of <1.035 were excluded from some analyses that examined the relationship of various environmental and biological factors and USG. Cats with USGs <1.035 but without identifiable pathology (either excluded or not pursued) were included in these analyses.
The effects of age, sex, diet, analysis method, fasting, drinking avidity, sampling time and visit reason on USG in apparently healthy cats were analyzed with a general linear model using the MIXED procedure of SAS. The outcome variable was modeled as a Gaussian variable and the assumption of normality was satisfied by visual inspection of the distribution of the studentized residuals. Because previous investigators have suggested that dietary management of feline lower urinary tract disease (FLUTD) might result in lower USG, and obstructive FLUTD is more commonly seen in male cats, we included a two-way interaction between diet and sex into the model. Variables were manually removed in a stepwise backward manner from the model when the P value was >0.05.
We further examined the factors that might be associated with an apparently healthy cat having a USG of <1.035. First, we dichotomized the outcome variable (USG <1.035 and USG ⩾1.035). The independent variables age, sex, diet, analysis method, fasting, drinking avidity, sampling time and visit reason were then fitted in a logistic regression model using the LOGISTIC procedure of SAS. Variables were manually removed in a backward stepwise manner beginning with the variable with the highest P value >0.05 until all remaining variables in the model were significantly associated with the outcome.
To examine whether older cats were more likely to have a pathological cause as an explanation for having a USG of <1.035, we compared the proportions of cats aged <9 years with cats aged >9 years and a USG of <1.035 that had identifiable disease on χ2 analysis.
Finally, to examine the effect of diet type on perceived drinking avidity, we performed ordinal logistic regression.
The significance for all statistical tests was set at P <0.05.
Results
Six hundred and ninety-two clinicians volunteered to participate in data acquisition; ultimately, data were submitted by 128 clinicians in first opinion practice.
Data were submitted for 993 adult cats and 64 young cats; 17 adult cats were excluded from further analysis because of presentation for inappropriate voiding. Demographics of all cats are presented in Table 1.
Table 1.
Characteristics of 976 apparently healthy adult cats and 64 apparently healthy young cats presenting to first opinion practice
| Adult cats |
Adult healthy cats |
Adult USG <1.035 |
Young cats (n = 64) | |
|---|---|---|---|---|
| (n = 976) | (n = 949) | (n = 121) | ||
| Age (years), median (range) | 6 (0.5–21) | 6 (0.5–21.0) | 11 (0.5–20.0) | 0.35 (0.15–0.45) |
| Sex, female (%) | 56 | 56 | 61 | 56 |
| Visit reason | ||||
| Annual examination or vaccination | 551 | 532 | 65 | 3 |
| Elective anesthesia | 409 | 402 | 54 | 61 |
| Minor medical | 16 | 16 | 2 | 0 |
| USG, median (range) | 1.050 (1.005–1.090) | 1.050 (1.005–1.090) | 1.025 (1.005–1.034) | 1.050 (1.017–1.080) |
USG = urine specific gravity
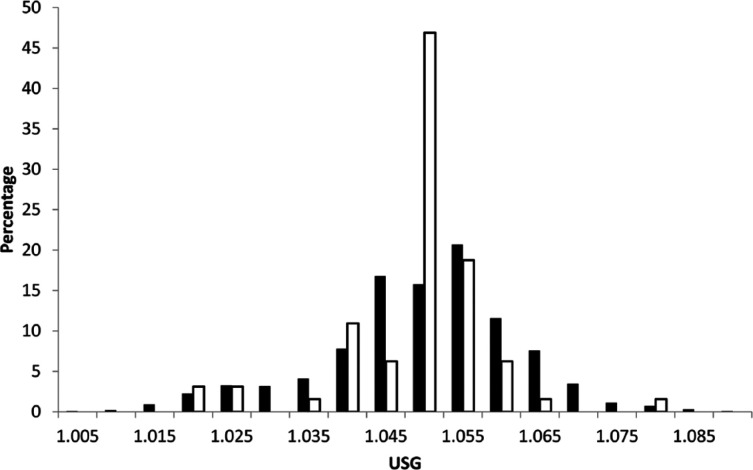
USG was ⩾1.030 in 91% (95% confidence interval [CI] 89–93%) and ⩾1.035 in 88% (95% CI 86–90%) of 976 adult apparently healthy cats; similar proportions were observed in young cats (94% ⩾1.030; 92% ⩾1.035). Additional investigation of the 121 adult cats and five young cats with USGs <1.035 (typically comprising a complete blood count, serum biochemistry and total thyroxine) identified a probable cause in 27 adult cats and no young cats. Of the 27 adult cats, only one cat was <9 years old (this cat was 9 months old). Causes in older adult cats consisted of chronic kidney disease and hyperthyroidism. No cause was identified in 43 adult cats, and a cause was not pursued in 51 adult cats. Clinicians remeasured USG in seven adult cats with initial USGs <1.035; five of these cats had USGs >1.035 on subsequent evaluation, while two cats had USGs <1.035, but no apparent clinical or biochemical abnormalities. Figure 1 shows the distribution of USGs in all apparently healthy young and adult cats. Significantly more cats ⩾9 years of age had a cause identified to explain a USG <1.035 than cats <9 years of age (P <0.01) (Figure 2).
Figure 1.
Distribution of urine specific gravity (USG) measurements in 976 apparently healthy adult cats and 64 apparently healthy young cats presenting to first opinion practice. Black bars represent all adult cats; white bars represent young cats
Figure 2.
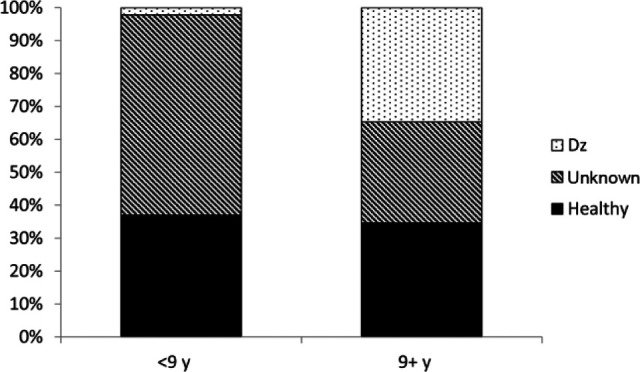
Proportions of adult cats older or younger than 9 years with identified pathology that could explain a urine specific gravity of <1.035. A cause was more commonly identified in older cats. Dz = Disease
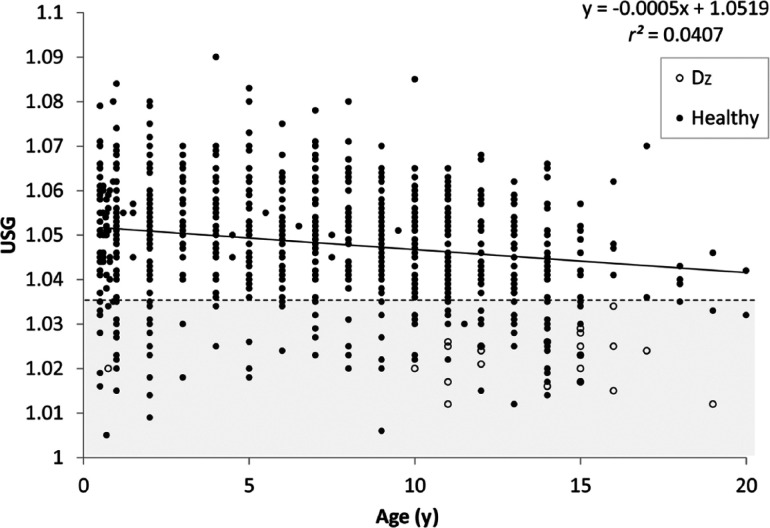
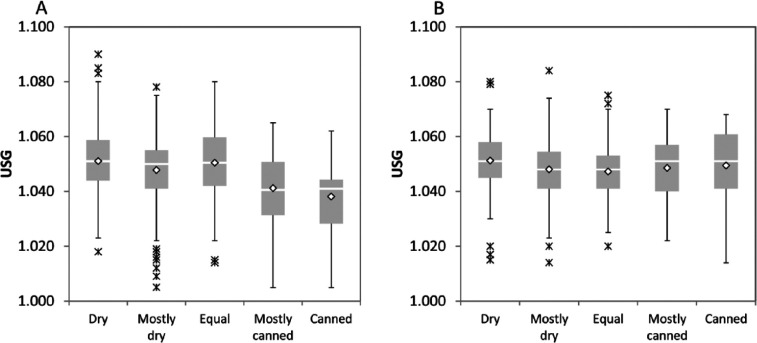
Factors that affected USG in the subgroup of 950 adult cats without subsequently identified disease included age (P <0.01), diet type (P <0.01), sex (P = 0.01), fasting status (P <0.01), drinking avidity (P <0.02), analysis method (P <0.01), and the interaction between diet type and sex (P <0.01). Specifically, USG decreased slightly with increasing age (Figure 3); decreased with increasing moisture content in the diet, but only in female cats (Figure 4a,b); was statistically lower in female cats than male cats (1.047 vs 1.049); was higher in unfasted cats than cats withheld either food or water prior to sampling (1.051 vs 1.047; data not shown); decreased with increasing drinking avidity (1.045 vs 1.041; Figure 5); and was higher in samples analyzed by reference laboratories than those analyzed with in-clinic refractometers (1.047 vs 1.051, data not shown).
Figure 3.
Relationship between age and urine specific gravity (USG) in 976 apparently healthy adult cats. Black circles represent apparently healthy cats without identifiable disease; open circles represent cats with identified pathology that could explain a USG of <1.035. The dashed line and shaded region encompasses USG <1.035. The regression equation was derived after excluding cats with pathology (open circles). Dz = Disease
Figure 4.
Box-and-whisker plots of urine specific gravity (USG) in cats fed different diets. (a) Female cats; (b) male cats. Gray boxes represent the interquartile range (IQR), horizontal lines represent the median, diamonds represent the mean, whiskers represent data within 1.5 × IQR of the median and asterisks represent data outside 1.5 × IQR of the median
Figure 5.
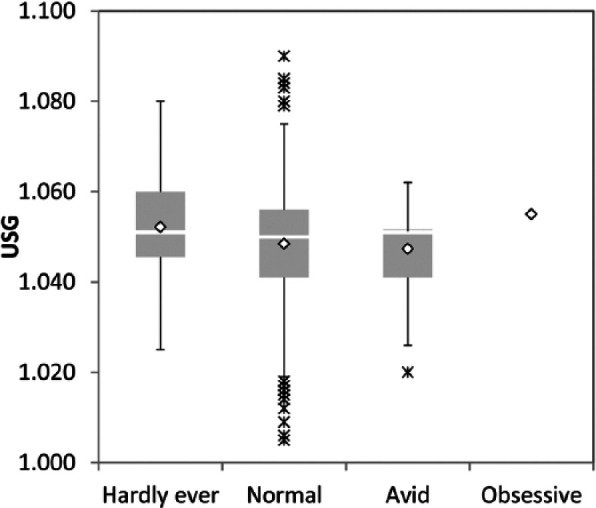
Box-and-whisker plots of urine specific gravity (USG) in cats with different drinking avidity. See Figure 4 for key
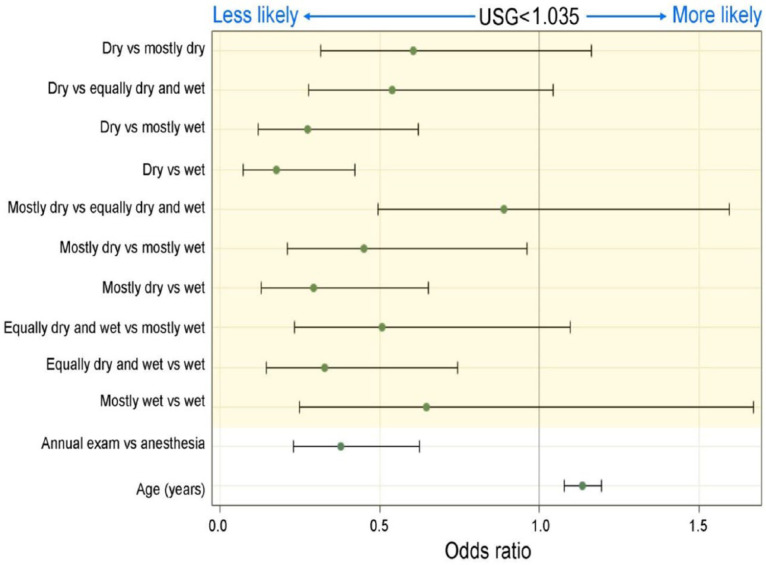
Logistic regression identified three factors that independently affected the odds of an apparently healthy adult cat having a USG of <1.035: age, visit reason and diet. Odds increased with age (P <0.01), were higher for cats presenting for elective anesthetic procedures compared with those presenting for annual wellness examinations (P <0.01), and increased with increasing moisture content of the diet (P <0.01) (Figure 6).
Figure 6.
Forest plot demonstrating factors contributing to the odds of an apparently healthy cat having a urine specific gravity (USG) of <1.035
Finally, cats decreased their drinking avidity with increasing dietary moisture content (P <0.01).
Discussion
Our large-scale study confirms the conventionally held wisdom, and reinforces observations from smaller studies, that apparently healthy adult cats, presenting for random urinalysis, generally have a USG of >1.035. Additionally, young cats (<6 months old) have a similar prevalence of USG >1.035. Unlike previous studies, our study also examined multiple environmental factors that might affect USG. We found that age, diet type, sex, fasting status, drinking avidity and analysis method all influenced USG. Furthermore, factors that increased the probability of having a USG of <1.035 in apparently healthy cats included increasing moisture content of the diet, age and reason for presentation to the first opinion practitioner (annual examination vs elective anesthetic procedure). Cats >9 years old had a higher probability of having a subclinical disease process identified as a cause of a USG of <1.035 than younger cats.
The association of increasing age with a decrease in USG was clinically unimportant, with most old cats still concentrating appropriately. However, older cats had a higher probability of having a USG of <1.035 than younger cats. In these cats, clinicians had a higher probability of identifying subclinical disease that might explain the low USG. However, this might reflect the willingness of a clinician to pursue a low USG in an older cat, and the ability of additional diagnostics – many of the young adult cats with USGs of <1.035 were shelter cats presenting for elective neutering or spaying, and could not be followed up by the clinician at a later date. However, in half of these young adult cats, the clinician failed to identify any underlying disease process – only one cat had a subsequent diagnosis of renal dysplasia. These data would suggest that clinicians should strongly consider additional diagnostics in an older, apparently healthy cat that has a random USG of <1.035. Conversely, extensive diagnostics in young cats are less likely to yield results consistent with a pathological etiology.
Many clinicians advise increasing the moisture content of the diet in cats either prone to, or suffering from, various feline lower urinary tract diseases. The basis for this recommendation is to dilute the urine through inadvertent increase in water consumption. 14 Some studies have demonstrated that cats consuming diets with high moisture content consumed more total daily water than when consuming dry diets, even though cats consuming dry diets drank more water.2,12,16 However, other studies have found no difference in total water consumption with different diets. 17 Some investigators also found that the USG of cats consuming dry diets was higher than that of cats consuming high moisture diets,2,16 while others found no difference in USG of (mostly male) cats consuming a canned ‘urolith prevention diet’ and a dry ‘urolith-forming diet’. 18 We found that dietary moisture content was associated with a slightly lower USG, but only in female cats. Twenty-five percent (13/62) of female cats fed largely or exclusively canned diets had a USG of <1.035 and 50% had a USG of >1.040. Conversely, 75% of male cats fed similar diets had a USG of >1.040, and only 3/49 (6%) male cats had a USG of <1.035. We could not explain the reason for selective effect of sex and diet type on USG. However, our data might suggest that dietary modification for controlling lower urinary tract disease in male cats might be less effective than in female cats. Clinicians should also note that most cats still had USGs of >1.040 regardless of diet moisture content, similar to observations of prior investigators. 18 However, diets higher in moisture content resulted in a higher probability of a cat having a USG of <1.035, an effect that was independent of sex or age.
Finally, we found an association of drinking avidity, as reported by the owner, and dietary moisture content – the higher the moisture content, the lower the reported drinking avidity. This would suggest that cats fed dry food supplement their water consumption by drinking, while those fed canned diets drink less, similar to observations of several previous studies.10,17 Some investigators observed that while voluntary water consumption was higher in cats fed dry foods, total water intake (voluntary and involuntary) was lower, suggesting that voluntary water consumption failed to compensate for the water content of the high-moisture diet. 10 Other investigators found that cats fed a dry diet consumed a similar amount of water to those fed a canned diet and did not differ in total body water, or body water turnover. 17 However, few studies examined the effect of the higher water consumption on USG.2,12,16,18 Our study would suggest that in cats outside of an experimental setting, overall water consumption might remain relatively stable, resulting in a USG of >1.035 in most cats, although the effect of diet in individual cats on USG would be best assessed by a prospective repeated measures study of cats afflicted with lower urinary tract disorders. Consequently, clinicians who alter dietary moisture content as a means of managing feline urinary tract diseases should confirm that the dietary modification has achieved the desired response by measuring both urine volume and USG, as previously suggested by other investigators. 14
Unexpectedly, fasting status affected USG in a counter-intuitive manner. Cats that were not fasted had a higher USG than those that were fasted (either completely, or had food withheld overnight). Fasted cats were more likely to be presented for elective anesthetic procedures than un-fasted cats. Similarly, cats presenting for elective anesthetic procedures were more likely to have a USG <1.035 than those presenting for routine annual evaluations. We could not find an explanation for these observations.
Equally unexpectedly, we found an association between visit reason and USG, independent of fasting status. We cannot provide a physiological explanation for this observation and believe it to be a false positive association (a type I error).
The USG also differed with analysis method – specifically, urine analyzed by reference laboratories had a higher USG than that analyzed by in-clinic refractometers. We did not observe differences between types of in-clinic refractometers (those with feline-specific scales vs those without). We did not do paired USG analysis to determine if the difference between methods was a function of method, sample handling or chance.
Similar to findings in a previous study, the sampling time did not affect USG. 3 In dogs, the ‘first morning’ sample is often considered the most concentrated, because most dogs sleep through the night without drinking, resulting in a higher USG in the first sample of the day. 1 However, cats are probably more nocturnal than dogs, and therefore drink or eat during the night, resulting in an early morning USG that is not different from that during the day.
Limitations
We did not measure total water consumption to determine if diet and drinking avidity affected total water intake and USG. Drinking avidity was based on a subjective assessment by the owner, without any standardization.
In almost 50% of young adult cats with a USG of <1.035, clinicians did not perform additional diagnostic tests to determine if a cause could be identified. In only a few cats did clinicians perform a repeat USG to determine if the initial measurement could be replicated. Therefore, the age-related probabilities of identifying disease in cats with USGs of <1.035 should be interpreted cautiously because of the potential bias in clinicians’ willingness to investigate potential causes.
Additionally, concentrating ability is often lost prior to any increases in biochemical analytes (blood urea nitrogen, creatinine). We did not have clinicians perform advanced renal function testing in cats where no underlying pathology could be identified; therefore, some of the apparently healthy cats without identifiable pathology might have subclinical renal dysfunction.
We did not request that all cats undergo additional biochemical testing to confirm health status. Thus, some apparently healthy cats with USGs >1.035 might have had unidentified occult diseases. Conversely, the ‘apparently healthy’ status was determined subjectively by the client and clinician, and would likely represent ‘apparently healthy cats’ presenting to other clinicians.
Finally, we only requested that participating clinicians calibrate their refractometers with distilled water (with a specific gravity of 1.000), but did not use quality control materials to assess the accuracy of the refractometers. However, this calibration method is similar to that used by other investigators. 19 Similarly, other studies have used refractometers to estimate USG in cats without detailing either the type of refractometer or calibration methods.4,7,9,10,18 Refractometers are generally considered robust instruments, and manufacturer data suggest that they are unlikely to become substantially uncalibrated over a 6 month period with routine use and care. While it is possible that some of the measurements might have been inaccurate, we would anticipate this inaccuracy to be random across the entire population, and would therefore not affect the group comparisons. However, a recent study has suggested that some in-clinic refractometers do not accurately measure solutions with USG in clinically relevant ranges, with measured specific gravity (SG) being lower than expected SG and the discrepancy increasing with increasing SG. 19 Therefore, it is not possible from our study (nor any study published to date) to determine the true USG of apparently healthy or diseased cats, but only to provide estimates obtained by clinical refractometry in practice. However, our results reflect the real-world findings of first opinion practitioners, rather than findings in a tightly controlled research setting, and are therefore broadly extrapolatable to the adult feline population.
Conclusions
Our study, in over 1000 apparently healthy adult cats, suggests that most cats concentrate their urine to a USG of >1.035 on a random single urinalysis. Dietary modification has a modest effect on USG, mostly evident in female cats. Older cats with USGs of <1.035 warrant additional investigation to determine if subclinical pathology is present that could account for the USG measurement.
Supplemental Material
Feline USG study data collection form
Acknowledgments
We would like to thank all the practitioners who collected urine samples, performed the USG analysis and submitted data to the study.
Footnotes
This study was presented as an abstract at the 2013 American College of Veterinary Internal Medicine Forum in Seattle, June 2013
Supplementary material: Feline USG Study Data Collection Form.
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific funding from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 3 June 2014
References
- 1. van Vonderen IK, Kooistra HS, Rijnberk A. Intra- and interindividual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Intern Med 1997; 11: 30–35. [DOI] [PubMed] [Google Scholar]
- 2. Holme DW. Research into the feline urological syndrome. In: Proceedings of the Kal Kan symposium for the treatment of dog and cat diseases; 1977 Sept 19–20; Ohio. Vernon, CA; Kal Kan, 1980, pp 40–45. [Google Scholar]
- 3. Finco DR, Adams DD, Crowell WA, et al. Food and water intake and urine composition in cats: influence of continuous versus periodic feeding. Am J Vet Res 1986; 47: 1638–1642. [PubMed] [Google Scholar]
- 4. Lees GE, Osborne CA, Stevens JB. Antibacterial properties of urine: studies of feline urine specific gravity, osmolality and pH. J Am Anim Hosp Assoc 1979; 15: 135–141. [Google Scholar]
- 5. DiBartola SP. Clinical approach and laboratory evaluation of renal disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, MO: Elsevier, 2010, p 1955. [Google Scholar]
- 6. Anonymous. Reference guides. Urine volume and specific gravity. In: Aiello SE. (ed). The Merck veterinary manual for veterinary professionals. Whitehouse Station, NJ: Merck. Available at: http://www.merckmanuals.com/vet/appendixes/reference_guides/urine_volume_and_specific_gravity.html#v3362553 (accessed June 30, 2014). [Google Scholar]
- 7. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paepe D, Bavegems V, Combes A, et al. Prospective evaluation of healthy Ragdoll cats for chronic kidney disease by routine laboratory parameters and ultrasonography. J Feline Med Surg 2013; 15: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoskins JD. Clinical evaluation of the kitten: from birth to eight weeks of age. Comp Contin Educ Pract Vet 1990; 12: 1215–1225. [Google Scholar]
- 10. Burger IH, Anderson RS, Holme DW. Nutritional factors affecting water balance in the dog and cat. In: Anderson RS. (ed). Nutrition of the dog and cat. Oxford: Pergamon Press, 1980, p 145. [Google Scholar]
- 11. Jackson OF, Tovey JD. Water balance studies in domestic cats. Fel Pract 1977; 7: 30–33. [Google Scholar]
- 12. Gaskell CJ. The role of fluid in the feline urological syndrome. In: Burger JH, Rivers JPW. (eds). Nutrition of the dog and cat. Waltham symposium 7. Cambridge: Cambridge University Press, 1989, p 353. [Google Scholar]
- 13. Thrall BE, Miller LG. Water turnover in cats fed dry rations. Fel Pract 1976; 6: 10–17. [Google Scholar]
- 14. Markwell PJ, Buffington CT, Smith BH. The effect of diet on lower urinary tract diseases in cats. J Nutr 1998; 128: 2753S–2757S. [DOI] [PubMed] [Google Scholar]
- 15. Westropp JL, Buffington CAT. Lower urinary tract disorders in cats. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis, MO: Elsevier, 2010, p 2069. [Google Scholar]
- 16. Buckley CM, Hawthorne A, Colyer A, et al. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br J Nutr 2011; 106 Suppl 1: S128–S130. [DOI] [PubMed] [Google Scholar]
- 17. Seefeldt SL, Chapman TE. Body water content and turnover in cats fed dry and canned rations. Am J Vet Res 1979; 40: 183–185. [PubMed] [Google Scholar]
- 18. Lulich JP, Osborne CA, Lekcharoensuk C, et al. Effects of diet on urine composition of cats with calcium oxalate urolithiasis. J Am Anim Hosp Assoc 2004; 40: 185–191. [DOI] [PubMed] [Google Scholar]
- 19. Tvedten HW, Noren A. Comparison of a Schmidt and Haensch refractometer and an Atago PAL-USG Cat refractometer for determination of urine specific gravity in dogs and cats. Vet Clin Pathol 2014; 43: 63–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Feline USG study data collection form