Fig. 5. HIV Env- and H1N1 NP-derived HLA-E-binding peptides elicit primary NK cell responses.
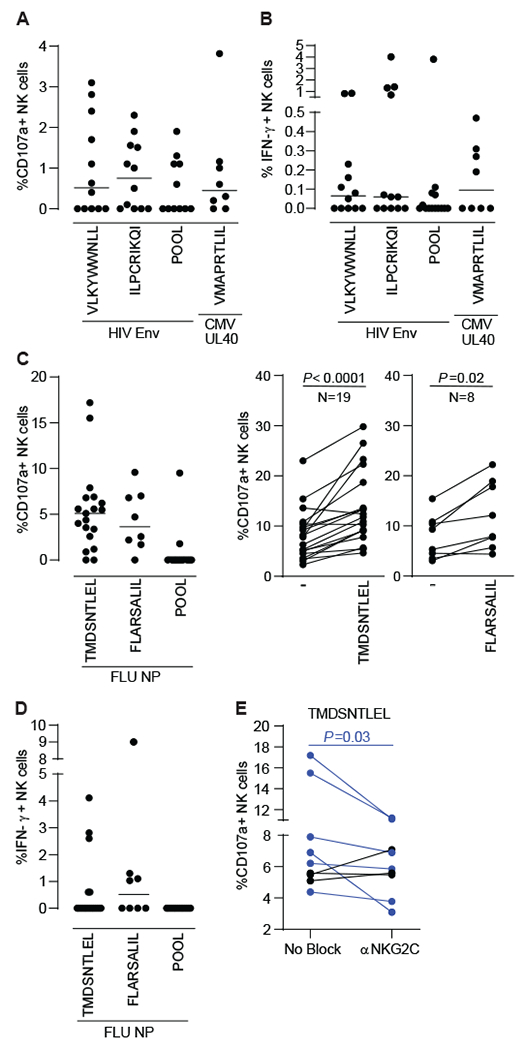
(A)(B) PBMCs samples from 12 PLWH (8 cART-treated and 4 untreated viremic) were incubated for 16h with 2μg/mL of 1 pool of 15 amino acid (aa) peptides overlapping by 11 aa and spanning HIV Env or single HIV Env- and CMV UL40-derived nonamers in the presence of CD107a antibodies. GolgiStop and GolgiPlug were added for the last 2 hours incubation prior to surface and intracellular cytokine staining to measure CD107a upregulation (A) and IFN-y production (B). Enriched NK cells from 19 healthy donors were incubated for 6 hours with 2μg/mL of 1 pool of 15 aa peptides overlapping by 11 aa and spanning H1N1 NP or 40uM of indicated single H1N1 NP-derived nonamers in the presence of CD107a antibodies, GolgiStop and GolgiPlug to evaluate CD107a upregulation (C) and IFN-γ production (D) by ICS. NK cell responses to H1N1 NP-derived TMDSNTLEL was evaluated in the presence of control or anti-NKG2C antibodies. Dot plots represent proportions of actively degranulating, cytotoxic NK cells, as judged by cell surface expression of CD107a (A, C left panel) or IFN-y producing NK cells (B, D) after subtracting proportions of CD107a+ and IFN-γ+ unstimulated NK cells, respectively. Bars represent the median. Paired plots compare CD107a expression between unstimulated and single-peptide-stimulated NK cells (C, right panels) or between NK cells stimulated with H1N1 NP-derived TMDSNTLEL in the presence of isotype control or anti-NKG2C antibodies after subtracting proportions of CD107a+ unstimulated NK cells (E). NK cells from all donors expressed NKG2C (PLWH: median 15.6%, range 2%-75%; healthy donors: median 10.3%, range 2%-48%). Statistical significance was tested using Wilcoxon signed-rank test (C and E).
