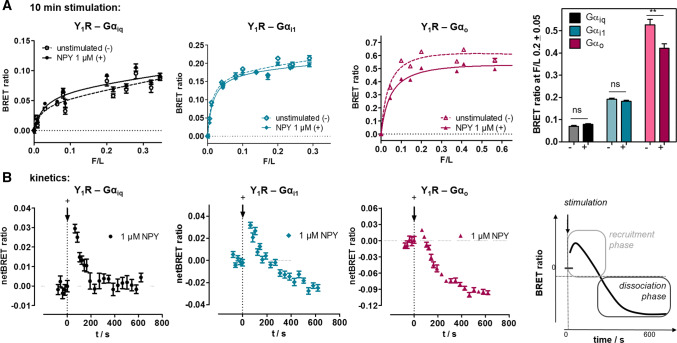
Fig. 5.
Pre-assembly and dissociation of Y1 receptor to inhibitory G proteins studied by saturation and kinetic BRET experiments. a Saturation BRET experiments of Y1-RLuc8 with different concentrations of chimeric Gα∆6qi4myr(Gαiq)-Venus, Gαi1-Venus or Gαo-Venus in transiently transfected HEK293 cells. BRET signal in the basal state in particular for Gαi1 and Gαio is saturable and indicates pre-assembly of the complex. Stimulation with 1 µM NPY for 10 min hardly changes BRET ratios for Gαiq but decreases BRET for Gαi1 and Gαio. Right panel: Comparison of BRET signal at a F/L ratio of 0.2 prior to stimulation (−) and after agonist stimulation for 10 min ( +). Columns are compared by two-tailed t-test. Shown is mean ± SEM of n ≥ 3 experiments conducted in technical quadruplicate. b Kinetic BRET experiments of the same constructs at saturating F/L ratio (F/L > 0.2) to resolve ligand effects on Y1R- Gα interactions. The BRET signal prior to stimulation was recorded for 2 min and the baseline was set to 0. After ligand stimulation (technical lag time 30 s), there was a short increase of the BRET signal, interpreted as recruitment, followed by a decrease of the BRET signal to (Gαiq) and below the baseline (Gαi1 and Gαio), interpreted as complex dissociation. Shown are buffer-corrected representative examples of n = 3 independent experiments conducted in technical quadruplicate