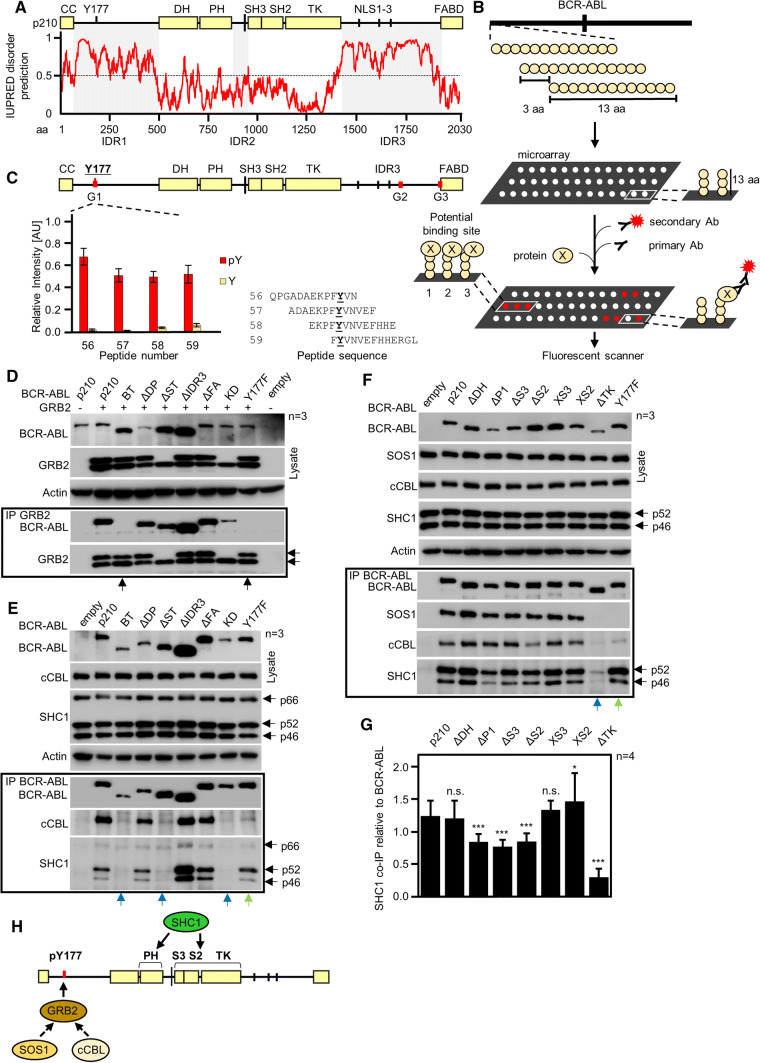
Fig. 5.
Interaction of GRB2, SOS1, cCBL, and SHC1 with BCR–ABL. a Secondary structure prediction of p210 BCR–ABL by IUPRED. Values above 0.5 indicate disordered regions IDR1 and IDR3 on the BCR–ABL N- and C-termini, involving Y177 and three NLS, respectively. Smaller disordered region IDR2 is located between domains PH and SH3. b Scheme of the microarray analysis. Thirteen amino acid long peptides corresponding to the primary sequence of p210 BCR–ABL were spotted on microarrays, incubated with protein of interest, primary and fluorescently labeled secondary antibodies, and scanned. Fluorescence intensity values for each spot were used to indicate the binding of protein to BCR–ABL peptides. c Microarrays indicate direct binding of GRB2 to phosphorylated Y177. Red lines on BCR–ABL scheme indicate potential binding sites. Graph shows averaged relative intensities for phosphorylated (red) and non-phosphorylated peptides involving peptides with Y177. Error bars indicate SD from three technical replicates shown in Fig. S2. d Co-immunoprecipitation (Co-IP) of BCR–ABL with GRB2 after expression in 293T cells; Y177F substitution abrogates GRB2 association with BCR–ABL as well as deleting the region (construct BT, bottom arrows). Side arrows indicate electrophoretic mobility shift GRB2 phosphorylated by BCR–ABL. e, f Co-immunoprecipitation of endogenous cCBL, SHC1 and SOS1 with transfected BCR–ABL in 293T cells. Please note the compromised SHC1 binding on BCR–ABL–BT, -ΔST, -ΔTK and KD variants (blue arrows). Y177F abrogates binding of SOS1 and largely limits the binding of cCBL (green arrows). Data are representative of three independent experiments (n = 3). g Quantification of SHC1 co-IP with BCR–ABL constructs from (f). SHC1 was normalized to BCR–ABL levels, error bars indicate SD from four independent experiments. Statistically significant differences are indicated (Student’s t test, *p < 0.05, ***p < 0.001; ns non-significant). h Scheme of the proposed interaction. GRB2 binds directly to phosphorylated Y177 and recruits SOS1. cCBL also requires GRB2 for recruitment. SHC1 requires TK domain and pleckstrin homology (PH) domain for binding