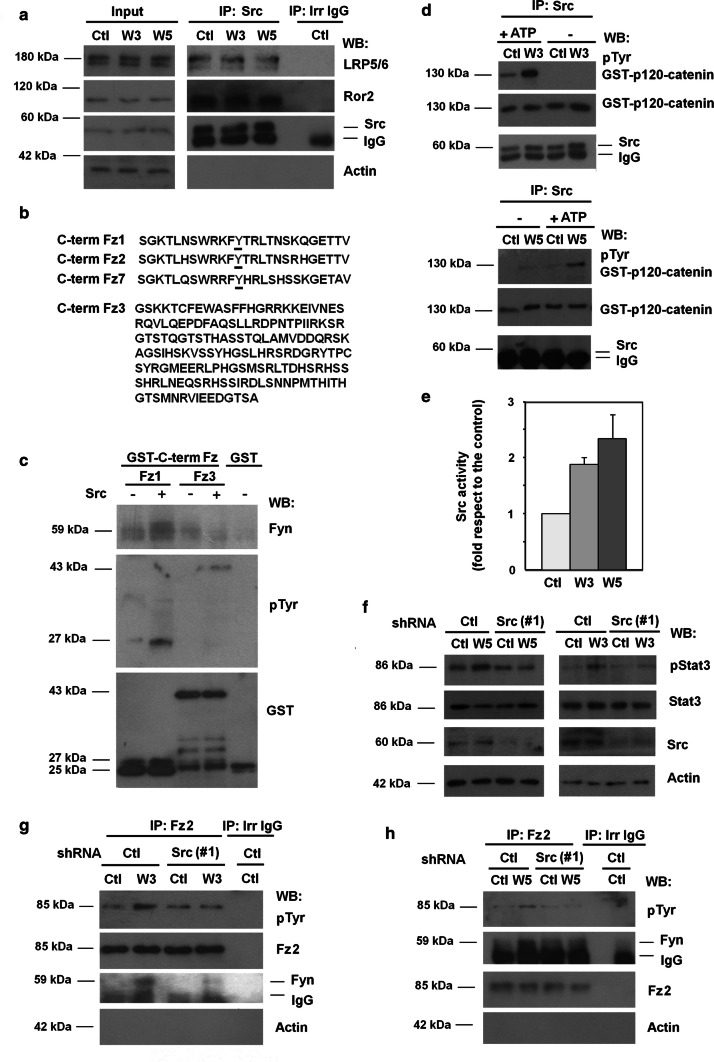
Fig. 3.
Wnt3a- and Wnt5a-dependent Src activation promotes Tyr552 Fz2 phosphorylation and binding to Fyn. a HEK293T cells were treated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min; Src was immunoprecipitated from total cell extracts, and associated co-receptors Ror2 and LRP5/6 were analyzed by WB. b Comparison of amino acid sequences of the C-terminal region of human Fz1, Fz2, Fz7 and Fz3. Underlined amino acid corresponds to Tyr552. c 4 pmol of recombinant GST-Fz1 and GST-Fz3 were in vitro phosphorylated by Src kinase. A pull-down assay was performed and associated Fyn and tyrosine-phosphorylated Fz was analyzed. d Src was immunoprecipitated from total cell extracts, and the immunocomplex was incubated with 8 pmol of recombinant GST–p120-catenin in Src phosphorylation conditions. Tyr phosphorylation in GST–p120-catenin was analyzed by WB as above. e Signal was densitometered, normalized with respect to the GST–p120-catenin and represented. The quantification of three different experiments is shown (mean ± SD). f–h HEK293T were transfected with shRNA control or shRNA Src(#1) and treated with control, Wnt3a- or Wnt5a-conditioned medium for 30 min, lysed and Stat3 phosphorylation was analyzed by WB (f). Fz2 was immunoprecipitated from total cell extracts treated with control, Wnt3a- (g) or Wnt5a-conditioned (h) medium; Fz2 tyrosine phosphorylation and Fyn recruitment to Fz2 were analyzed by WB