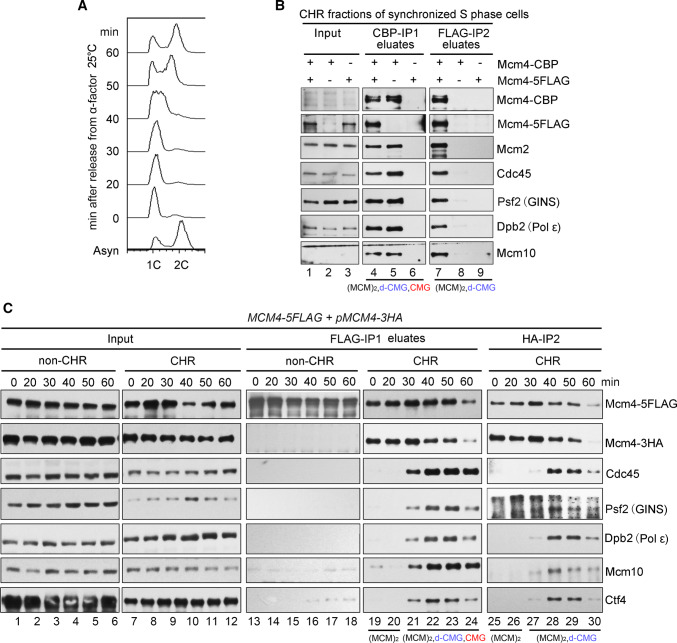
Fig. 1.
Identification of a CMG-containing complex harboring two MCMs. a A representative cell cycle profile of the samples used for protein complex purification. Cells (Strain LL45-1, Table S1) were grown in dropout media at 25 °C and synchronized by 7.5 μg/ml α factor. G1 arrested cells were released and continued growth for the indicated time. Cell cycle profiles were analyzed by flow cytometry. b The MCM4-CBP/pMCM4-5FLAG (Strain LL94-1, Table S1) cells were grown, synchronized in G1 by α-factor, and released into S phase at 25 °C for 40 min. The chromatin-bound protein fraction (CHR) was prepared and subjected to tandem affinity purification via calmodulin and anti-FLAG M2 resins. After three washes, the bound fractions were eluted from beads by 3 mM EGTA (labeled as CBP-IP1 eluates) and 2 mg/ml FLAG peptides (labeled as FLAG-IP2 eluates), respectively. The eluted samples were resolved on an SDS-polyacrylamide gel (PAGE) and detected via immunoblots using the indicated antibodies. Strains (LL94-2 and LL6-1, Table S1) harboring a single tag (either CBP or 5FLAG) on Mcm4 were applied as controls. The species of MCM-containing complexes obtained after each purification step are indicated for clarity. c The MCM4-5FLAG/pMCM4-3HA (LL45-1, Table S1) cells were grown, synchronized in G1 by α-factor (0 min) and released into S phase at 25 °C for the indicated time. Spheroplasts were fractionated into the non-chromatin-bound (non-CHR) and chromatin-bound (CHR) protein fractions. Mcm4-5FLAG and then Mcm4-3HA were precipitated consecutively in a similar procedure mentioned above. After three washes, the proteins specifically associated with beads were eluted by 2 mg/ml of FLAG peptide or boiled directly (for HA-IP) before western blotting. The species of MCM-containing complexes obtained after each purification step are indicated for clarity