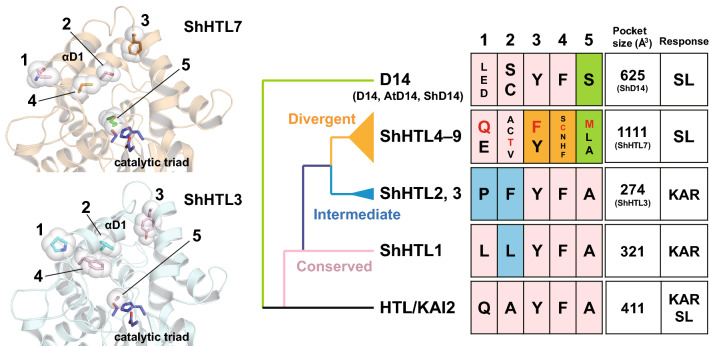
Fig. 3.
Structural basis of the molecular evolution of ShHTLs. The catalytic pocket is narrowed by the residues in the blue box (positions 1 and 2 of the intermediate clade and position 1 of the conserved clade) to prevent SL binding. The residue at position 1 affects the arrangement of helix αD1 on the helical cap domain. The residues in the orange box (positions 3 and 4 of divergent clade) contribute to expanding the catalytic pocket to accept various types of SLs. D14 and ShHTLs of the divergent clade are not capable of binding KAR due to steric hindrance with the residues in the green box (position 5). The residues of ShHTL3 and ShHTL7 (colored in red) are shown on their structure models