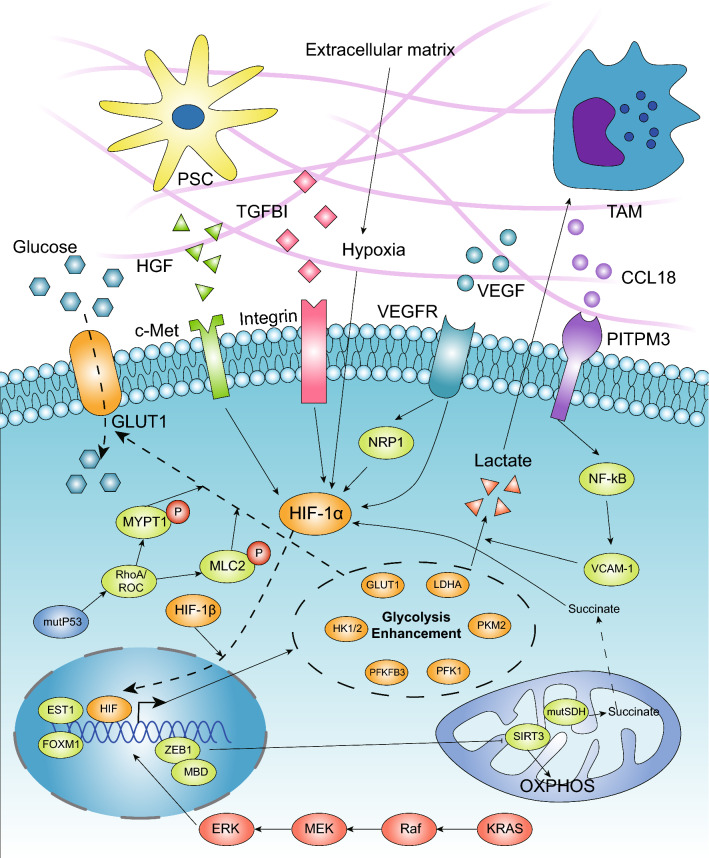
Fig. 2.
Driver genes and TME promote the enhancement of glycolysis in PDAC. The high-glycolysis phenotype is considered to be an emerging hallmark associated with activated oncogenes. Mutant Kras can upregulate the expression of glycolytic enzymes via the Raf/MEK/ERK pathway, which is dependent on Myc and functions transcriptionally. GOF mutp53 also promotes glycolysis mainly by regulating GLUT1 translocation to the cytoplasmic membrane. In the pancreatic microenvironment, hypoxia is considered the main regulator that transcriptionally upregulates the expression of multiple glycolytic enzymes. SDH mutations leading to succinate accumulation can promote glycolysis through stabilizing HIF-1α. Additionally, stroma cells, such as TAMs and pancreatic stellate cells, secrete cytokines that interact with membrane receptors in cancer cells, and soluble factors in the TME, such as VEGF and TGFBI, promote glycolysis mainly via the HIF-1α and NF-kB pathways. ZEB1, known as an EMT regulator, can also promote glycolysis via its interaction with MBD1, which transcriptionally suppresses the expression of SIRT3