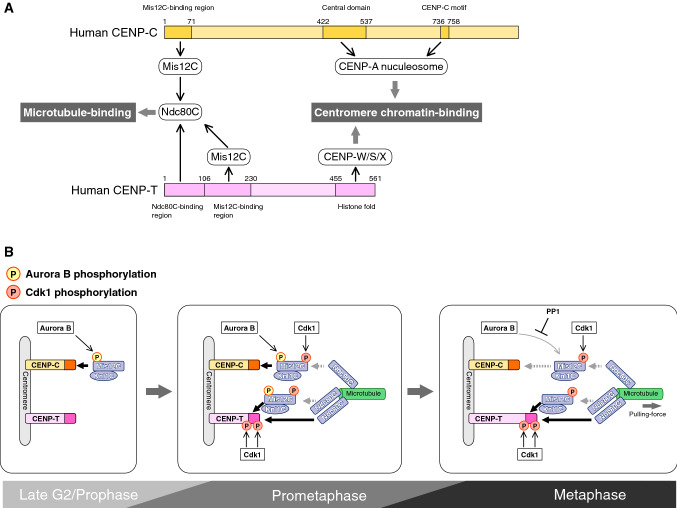
Fig. 7.
Two-pathway links between centromere chromatin and spindle microtubules. a Two-pathway: CENP-C and CENP-T pathways. CENP-C has a Mis12 complex-binding (Mis12C-binding) region in its N terminus and two CENP-A nucleosome-binding domains in its middle and C-terminal regions (central domain and CENP-C motif, respectively). CENP-T binds to the Ndc80 complex (Ndc80C) and Mis12C via its N-terminal region. The CENP-T C terminus has a histone fold domain, which forms a nucleosome-like structure with CENP-W, -S, and -X, binding to centromere chromatin. Both CENP-C and CENP-T recruit to Ndc80C, which binds to spindle microtubules directly or indirectly through Mis12C, and also interacts with the centromere chromatin, forming bridges between the centromere and the microtubules. b Dynamic interaction of the KMN network with the two pathways. Two CCAN components, CENP-C and CENP-T, interact with and recruit the KMN network to the centromere in M-phase. The multiple phosphoregulations by CDK1 and AurB induce changes in the interaction between CCAN and the KMN network during M-phase progression, making the CENP-T pathway the major load-bearing KMN scaffold