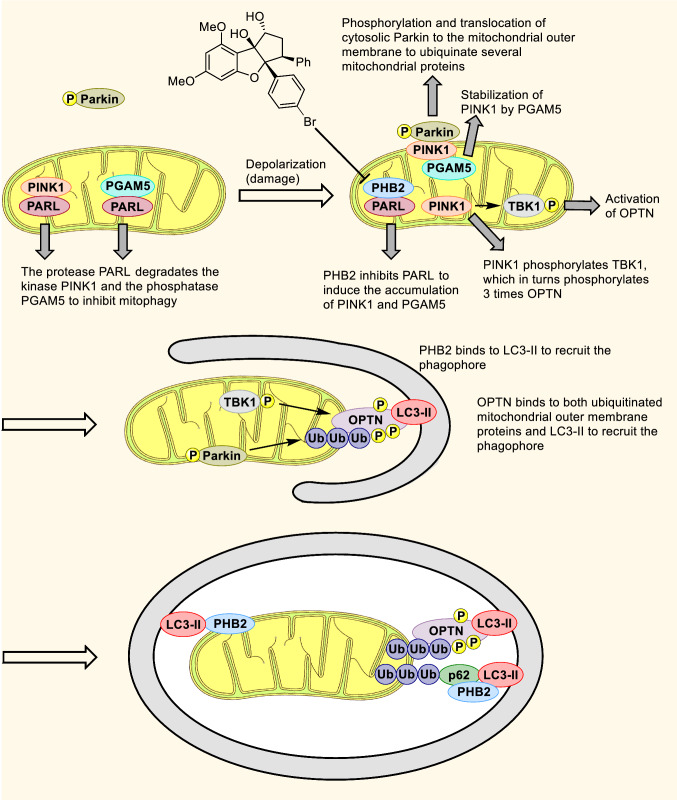
Fig. 6.
Regulation of mitophagy by PHB2. In healthy mitochondria the protease PINK1 and the phosphatase PGAM5 are continuously imported and degraded by the protease PARL. Upon damage, PHB2 interacts with PARL to block its activity leading to accumulation of uncleaved PINK1 on the outer mitochondrial membrane. Then PINK1 phosphorylates the ubiquitinated chains conjugated to mitochondrial proteins as well as cytosolic Parkin and the kinase TBK1 to induce their activation. TBK1 phosphorylates 3 times Optineurin (Optn). Autophagy receptors, PHB2, Optn, and p62 interact with LC3-II to recruit the phagophore and promote its expansion. Flavaglines inhibit the interaction between PHB2 and PARL, leading to an inhibition of mitophagy [76]. At the mitochondrial inner membrane, PHB2 forms ring-like oligomers with PHB1, but a role of PHB1 in mitophagy has not been established yet