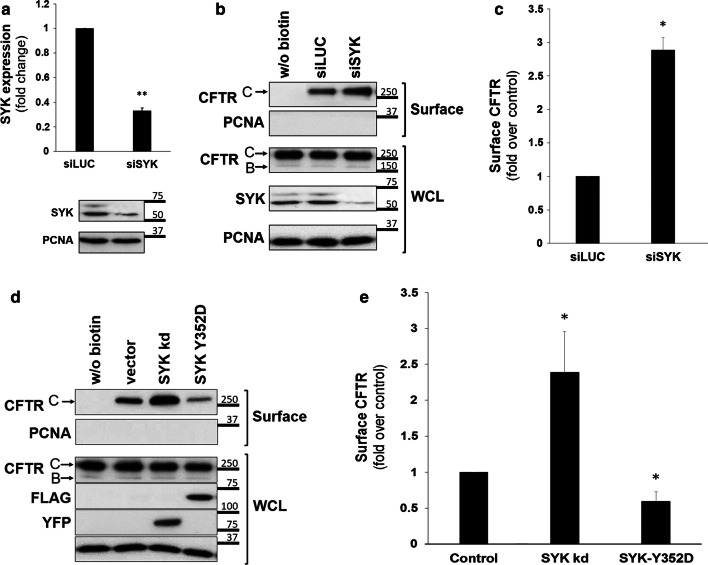
Fig. 2.
Effect of experimental manipulation of SYK expression levels and its catalytic activity on the levels of CFTR at the PM of CFBE wt-CFTR cells. a Assessment of the efficiency of siRNA-mediated knockdown of SYK. CFBE wt-CFTR cells were transfected as described under “Cell culture and transfections”and depletion of endogenous SYK protein levels analyzed by Western blot. Data quantification by Student’s t test (n = 6) is graphically displayed and shown as fold-change relative to siLUC (control). Note the successful downregulation of SYK expression by approximately 70%. b, c Effect of depleting endogenous SYK expression in CFBE wt-CFTR cells transfected with one of the indicated siRNAs. d, e Effect of overexpression of SYK mutants in cells transfected with empty-vector, inactive mutant YFP-SYK kd, or constitutively active mutant Flag-SYK Y352D. After the treatments described above, cell surface proteins were biotinylated, cells were lysed, and proteins resolved by SDS-PAGE and detected by WB. b, d Detection of the indicated proteins in whole-cell lysates (WCL) or in the biotinylated protein fraction (surface). For CFTR, the mature, fully glycosylated and the immature, core-glycosylated protein are marked as bands C and B, respectively. The PCNA protein served as a loading (WCL) and contamination (surface) control, respectively. c, e Corresponding quantification of CFTR detection in the biotinylated cell surface fractions, obtained from at least three independent experiments. a, c, e Densitometric analyses of the band intensities were expressed relative to the control sample (n ≥ 3) and analyzed either by unpaired Student’s T tests (a, c) or one-way ANOVA (P < 0.001), followed by post hoc Tukey’s tests (e). All shown data represent means ± SEM. *P < 0.05; **P < 0.01