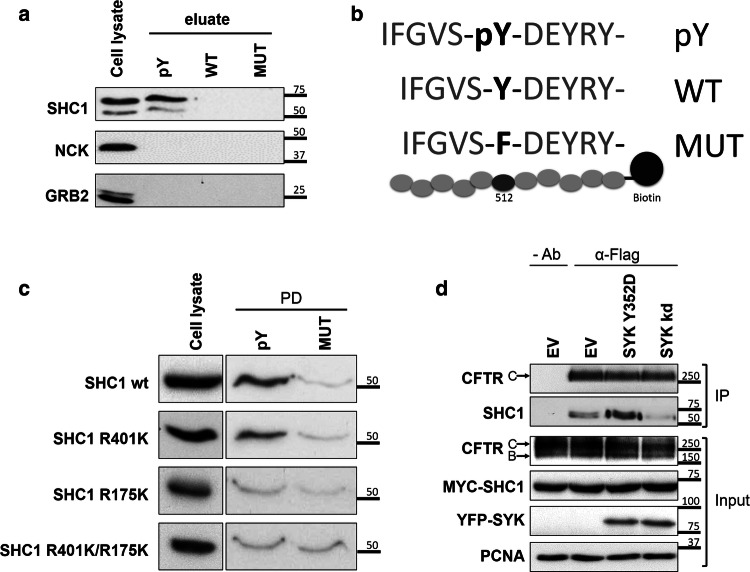
Fig. 4.
Validation of the interaction of SHC1 with tyrosine-phosphorylated CFTR. a WB analysis of the protein fraction isolated from CFBE wt-CFTR cell lysates using an affinity peptide pull-down (PPD) assay with the indicated synthetic peptides. Note the specific binding of both endogenous SHC1 isoforms to the tyrosine-phosphorylated CFTR peptide, whereas two other SH2 domain containing proteins (GRB2, NCK) did bind the CFTR peptides. pY—phosphorylated peptide; WT—non-phosphorylated peptide; MUT- peptide with substitution of tyrosine by phenylalanine. b Graphic display of the three CFTR peptide sequences (pY, WT and MUT) used for the PPD assay. c The PTB domain of SHC1 is required for association with CFTR phosphotyrosine domain. WB analysis following the PPD assay with lysates from CFBE wt-CFTR cells previously transfected with the indicated SHC1 mutants. Note that the point mutation R175K in the SHC1 PTB domain abolishes binding to the phosphorylated peptide. d SHC1 forms a protein complex with CFTR depending on SYK protein kinase activity. CFBE mCherry-Flag-wt-CFTR cells were co-transfected with Myc-SHC1 and either constitutively active SYK Y352D or SYK kd mutants. The fraction of PM-localized CFTR was immunoprecipitated with anti-Flag antibody (IP) and the amount of SHC1 co-precipitating with CFTR analyzed by WB. Note that the presence of active SYK strongly increased the co-precipitation of SHC1 with the mature, fully glycosylated CFTR-band C. Input- whole cell lysates; IP- immunoprecipitated fractions; Ab- antibody. All data shown are representative for at least three independent experiments