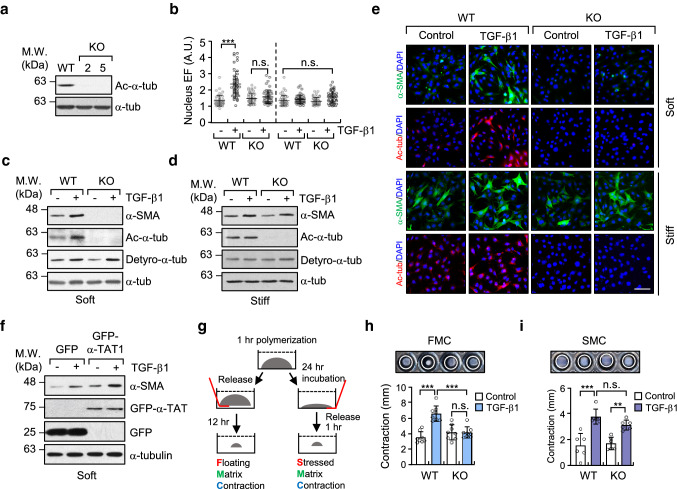
Fig. 2.
Increase in microtubule acetylation is required for TGF-β1-induced myofibroblast differentiation on soft matrix. a Western blotting conducted to detect the level of acetylated-α-tubulin in α-TAT1 KO MEFs (clone #2 and 5) established using a CRISPR/Cas9 system. b Quantification of nuclear elliptical factor (EF) in WT and α-TAT1 KO MEFs upon treatment with TGF-β1 for 8 h. Data are represented as mean ± S.D. from two independent experiments (n = 50). Statistical analysis was performed by one-way ANOVA with Tukey’s multiple comparisons. One-way ANOVA, F7, 392 = 34.74. c, d Western blotting of α-SMA in WT and α-TAT1 KO MEFs grown on soft (c) and stiff (d) matrices. e Immunofluorescence images of α-SMA (green) and acetylated-α-tubulin (red) in WT and α-TAT1 KO MEFs upon stimulation with TGF-β1. Scale bar, 100 µm. f MEFs were transfected with GFP or GFP-α-TAT1 for 24 h. After serum starvation for 12 h, cells were seeded on fibronectin-coated soft matrix and stimulated with TGF-β1 for 8 h. Cells were lysed and subjected to western blotting using antibodies specific for α-SMA and GFP. g Schematic diagram of 3D collagen matrix remodelling assays (FMC, floating matrix contraction; SMC, stressed matrix contraction). h, i WT and KO cells were embedded in 3D matrices composed of 1 mg ml−1 collagen and 100 µg ml−1 fibronectin, and incubated for 1 h. After adding TGF-β1 into each matrix, FMC (h) and SMC (i) assays were performed as shown in (g). Graphs show the reduced size of the 3D collagen gel compared with the original gel size. Data are represented as the mean of four independent experiments ± S.D. (n = 8, each group). FMC: one-way ANOVA, F3, 28 = 19.59, SMC: one-way ANOVA F3, 20 = 17.91. p value p < 0.05 considered being significant. **p < 0.01, ***p < 0.005, n.s. not significant