Abstract
As the dominant constituent of the extracellular matrix (ECM), collagens of different types are critical for the structural properties of tissues and make up scaffolds for cellular adhesion and migration. Importantly, collagens also directly modulate the phenotypic state of cells by transmitting signals that influence proliferation, differentiation, polarization, survival, and more, to cells of mesenchymal, epithelial, or endothelial origin. Recently, the potential of collagens to provide immune regulatory signals has also been demonstrated, and it is believed that pathological changes in the ECM shape immune cell phenotype. Collagens are themselves heavily regulated by a multitude of structural modulations or by catabolic pathways. One of these pathways involves a cellular uptake of collagens or soluble collagen-like defense collagens of the innate immune system mediated by endocytic collagen receptors. This cellular uptake is followed by the degradation of collagens in lysosomes. The potential of this pathway to regulate collagens in pathological conditions is evident from the increased extracellular accumulation of both collagens and collagen-like defense collagens following endocytic collagen receptor ablation. Here, we review how endocytic collagen receptors regulate collagen turnover during physiological conditions and in pathological conditions, such as fibrosis and cancer. Furthermore, we highlight the potential of collagens to regulate immune cells and discuss how endocytic collagen receptors can directly regulate immune cell activity in pathological conditions or do it indirectly by altering the extracellular milieu. Finally, we discuss the potential collagen receptors utilized by immune cells to directly detect ECM-related changes in the tissues which they encounter.
Keywords: Collagen endocytosis, Collagen receptors, ECM remodelling, Immune regulation, Fibrosis, Cancer, Defense collagens, Mannose receptor, uPARAP
Introduction
Tissue injury, resulting from trauma or disease, leads to numerous actions of immune and inflammatory cells in a complicated interplay with the resident non-immune cells and the extracellular matrix (ECM) of the tissue in question. Depending on the type of injury, local conditions, and systemic factors, these actions may contribute to injury repair or, conversely, they may exacerbate conditions through increased and sustained inflammation.
Since the ECM acts as a physical support of tissues and also takes part in the regulation of several cellular processes, alterations in the ECM strongly influence the consequences of injury. In many cases, the ECM becomes mechanically or enzymatically degraded, followed by cellular uptake of degradation products. In other cases, a net accumulation of ECM is part of the pathogenic events, leading to a central role of matrix re-uptake processes that serve to counteract the excessive deposition [1].
The structural collagen types are the most abundant matrix constituents and their complicated pathways of degradation and cellular uptake are in the core of ECM-associated pathological processes. The main focus of this article will be on processes related to the interstitial collagens, which include collagen types I, II, and III [2]. We first review a number of features of the various collagen uptake processes and their associated receptors, and highlight the influence which these receptors have on collagen levels in the ECM in pathological conditions such as cancer and fibrosis. By drawing on observations made in published in vitro studies and in studies of ECM-related diseases, we then discuss the implications of these collagen-degrading mechanisms in regulating cellular functions, with a strong focus on the control collagen can impose on the phenotypes of immune cells.
Collagen turnover in homeostasis and tissue injury
The turnover of collagens in tissues such as bone is not restricted to injury or pathological conditions but is also part of a healthy steady state condition with de novo deposition of collagen balancing collagen turnover. In both homeostasis and tissue injury, the same enzymatic and receptor-mediated collagen turnover processes seem to be operative. Therefore, the major difference between homeostatic and trauma-associated collagen turnover lies in the net balance between synthesis and turnover [3].
The initial cleavage of collagens in the extracellular environment is undertaken by specialized members of the matrix metalloprotease (MMP) family and, in the bone compartment, by osteoclast-derived cathepsin K. These proteolytic processes have been treated in several recent reviews (e.g., [4, 5]) and will not be covered in detail here. The cell types responsible for collagen degradation differ from tissue to tissue and between different physiological and pathological conditions. In most cases, fibroblasts, macrophages, or specialized tissue-resident cells with a close relation to either fibroblasts or macrophages dominate. For instance, during homeostasis of skin and cartilage, both of which are tissues very rich in interstitial collagens, fibroblasts, or the fibroblast-like chondrocytes constitute the key players in collagen degradation [6–8]. Likewise, osteoclasts, which derive from progenitors of a monocyte/macrophage hematopoietic lineage, constitute the major collagen-degrading cell type in bone homeostasis [9]. In pathologies such as cancer, collagen degradation is mediated by the concerted action of cancer cells and stromal cells, but with stromal fibroblasts and macrophages being the major contributors [10, 11]. In other pathologies with a more anatomically distinct appearance, cell types not related to macrophages and fibroblasts may, however, also facilitate collagen degradation. Examples of these include endothelial cells that facilitate collagen II degradation in the vitreous humour of the eye in proliferative diabetic retinopathy [12, 13] and vascular smooth muscle cells which degrade collagen III in the medial layer of the walls of blood vessels, when infiltrating the subendothelial space where they can form neointimal scar tissue [14, 15]. In the following, we will focus on the cellular collagen uptake mechanisms that constitute a crucial part of the degradation machinery, but which have been subject to less investigation (Fig. 1).
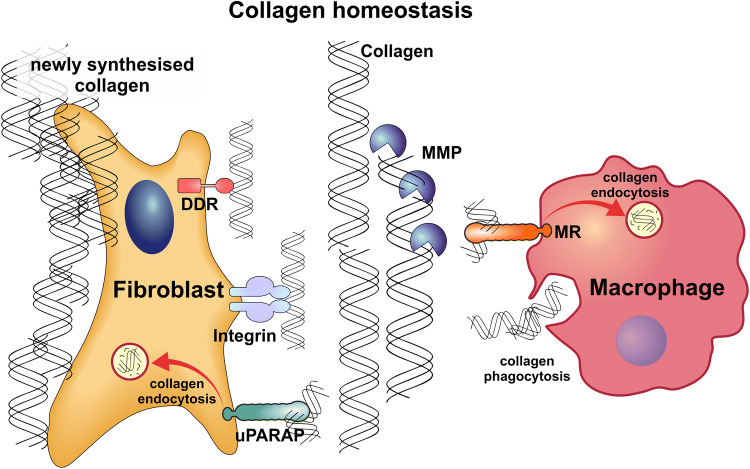
Fig. 1.
Collagen homeostasis is governed by cellular uptake of collagen, extracellular collagenolysis, and synthesis of new collagen. Fibroblasts and macrophages are the principal cell types engaged in the maintenance and remodelling of collagen structures in the ECM. Collagens are broken down by extracellular proteases produced by these cells. Most of the proteases that degrade collagens belong to the MMP protein family. Larger pieces of collagen are taken up by macrophages through phagocytosis. Pre-fragmented collagens are endocytosed by macrophages via MR and by fibroblasts via uPARAP. Collagens engulfed through phagocytosis or taken up via receptor-mediated endocytosis are subsequently degraded in lysosomes through the action of cysteine cathepsins. Fibroblasts also constitute the main collagen-producing cells. Discoidin Domain Receptors (DDRs) and β1-integrins constitute other known collagen receptors, but no role of these receptors in collagen homeostasis has been described
Two major pathways of intracellular collagen degradation have been identified: (1) a phagocytic uptake of particulate collagen and (2) a receptor-mediated endocytosis of pre-solubilized collagen. Both of these pathways are likely to be preceded by one or several steps of collagen cleavage prior to collagen internalization. Importantly, however, collagen uptake is still a rate-limiting factor in turnover, because, in its absence, undigested collagen material accumulates in the extracellular space [16, 17].
Collagen phagocytosis
Fibroblasts are one of the principal cell types engaged in the phagocytosis of collagens. Thus, collagen fibrils, phagocytosed from the extracellular space, can be observed in fibroblasts by electron microscopy [18]. Collagen-coated beads have been employed to study various details of the phagocytic process in this cell type. In gingival fibroblasts, this process is strongly dependent on the α2β1 integrin [19, 20], in accordance with the well-defined binding of collagen to this integrin [21]. Studying the same cell type, MT1-MMP was found to be the major protease responsible for collagen cleavage preceding phagocytosis [22].
The collagen-binding proteoglycan, decorin, has some regulatory function in collagen phagocytosis, since increasing the binding of decorin to collagen leads to a decrease in the integrin-dependent phagocytosis of collagen-coated beads [23]. The role of fibronectin is less clear. The α2β1 integrin-dependent, cellular uptake of collagen has indeed been shown to be increased under conditions where fibronectin-mediated stabilization of collagen I fibrils was inhibited [24], but this effect might both be related to collagen phagocytosis and to the receptor-mediated endocytic process described below.
Receptor-mediated collagen endocytosis
Clathrin-dependent endocytosis of collagens involves well-defined endocytic receptors with collagen-binding specificity of which two have been identified. These receptors are the urokinase plasminogen activator receptor-associated protein (uPARAP/Endo180), expressed on several mesenchymal cell types and some macrophages, and the mannose receptor (MR, CD206), expressed on macrophage and dendritic cell subsets, and certain endothelial cells. uPARAP/Endo180 and MR are type-1 transmembrane proteins with a very similar domain organization [25–28], and they both bind to collagens through their fibronectin type-II domain, the second domain from the NH2-terminus [29, 30].
uPARAP/Endo180 is expressed on activated fibroblasts during processes of ECM remodelling [17, 31] and on certain osteoblast-like cells, including lining cells [32, 33]. It is a constitutively recycling, clathrin-associated receptor, delivering its cargo to the endosomal system [34]. uPARAP/Endo180 efficiently endocytoses collagens of many or all subtypes in a process that ultimately leads to lysosomal degradation of the endocytosed collagen and recycling of unliganded uPARAP/Endo180 to the cell surface [35–39]. Since the clathrin-dependent route of endocytosis would not allow the internalization of large collagen particles, one or several collagen cleavage steps must precede this cellular uptake. Accordingly, fibroblast-expressed MT1-MMP can act on a polymerized collagen matrix to generate fragments that are efficiently internalized by uPARAP/Endo180 for intracellular degradation in the same cells [40]. Although this consecutive reaction pattern resembles that observed for the phagocytic process above, these pathways are clearly distinct. Thus, uPARAP/Endo180 is not involved in collagen phagocytosis in fibroblasts [41, 42].
Although uPARAP/Endo180-deficient mice are viable and fertile [35, 36], deficiency of this receptor leads to a minor delay of bone growth in mice [43, 44]. In contrast, a pronounced bone defect is observed in “Belgian Blue” cattle carrying a null mutation in uPARAP/Endo180 [45, 46], suggesting that the impact of uPARAP/Endo180-loss is species-specific. No human pathological condition has been ascribed to mutations in the uPARAP/Endo180 gene so far.
MR is a multi-ligand receptor, which can bind and internalize collagen in a manner very similar to that of uPARAP/Endo180 [29, 47], and indeed, they are receptors belonging to the same protein family. Although this protein family includes a total of four endocytic receptors, uPARAP/Endo180 and MR are the only collagen receptors in it [30].
The role of uPARAP/Endo180 and MR in collagen uptake and their relative contribution to this process in vivo has been investigated in several studies. When heat-denatured collagen is introduced into the blood stream of mice, this material is cleared by liver sinusoidal endothelial cells in a process that is strictly dependent on MR [48]. Collagen uptake has also been studied in detail in the skin [49, 50]. Here, M2-like macrophages were identified as a major cell type in collagen uptake, with a smaller contribution from fibroblasts, and both MR and uPARAP/Endo180 could be shown to contribute to the collagen clearance process, being expressed by distinct subpopulations of macrophages.
Collagen uptake in disease
Several human diseases feature pronounced perturbations of collagen homeostasis, and in some of these, a net accumulation or an excessive degradation directly contributes to the pathogenesis.
Fibrosis
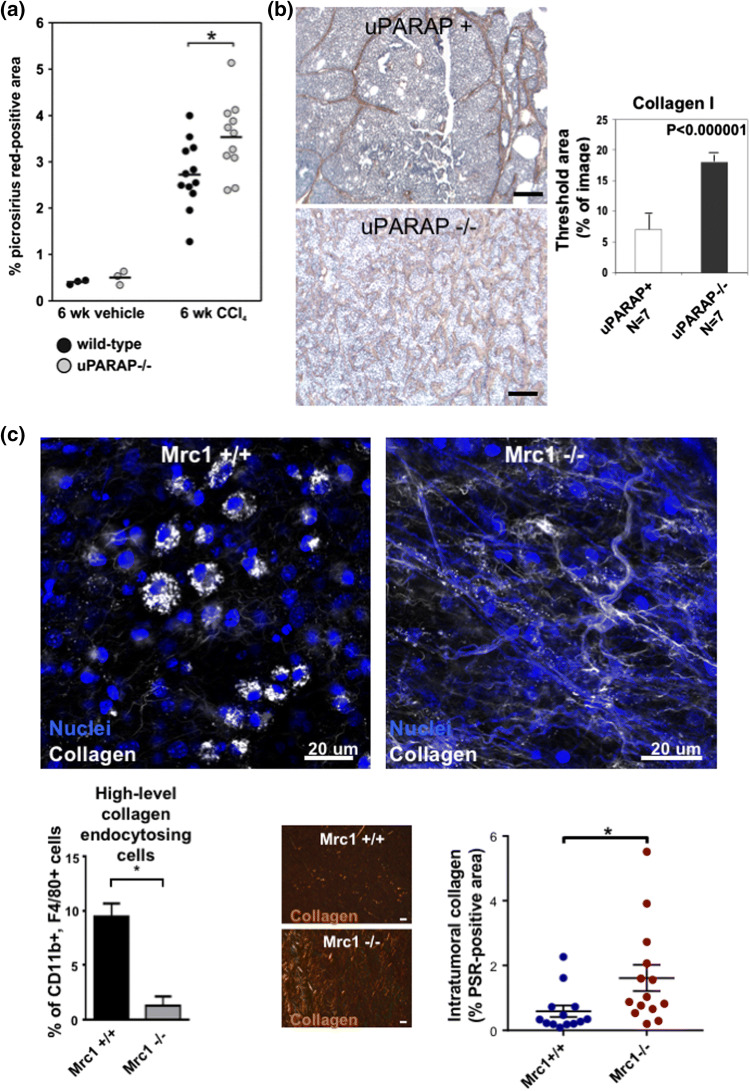
Collagen deposition is a hallmark of fibrosis, and in several fibrotic conditions, the extent of collagen uptake turns out to be critical for disease severity. In human cirrhotic livers, as well as in mice in which liver fibrosis was induced by repeated exposure to CCl4, a strong upregulation of uPARAP/Endo180 was observed in hepatic stellate cells and in activated fibroblasts adjacent to collagen deposition [17]. This upregulation of uPARAP/Endo180 was functionally important, as an increased net deposition of collagen was observed after ablation of the uPARAP/Endo180 gene (Fig. 2a). Similar studies in mice have shown that uPARAP/Endo180 is upregulated in interstitial myofibroblasts in renal fibrosis, where it serves to counteract collagen deposition in the same manner, as shown in gene inactivation studies [51]. In the mouse lung, after treatment of mice with bleomycin, the accumulation of collagen was likewise enhanced in uPARAP-deficient mice, but in this case, parameters such as alveolar permeability to fluorescent dextran were also affected by uPARAP/Endo180 deficiency [52]. The alveolar macrophage glycoprotein, milk fat globule epidermal growth factor 8 (Mfge8), can bind to collagen accumulations in the lung and has been found to facilitate the re-uptake of this material, in this case undertaken by macrophages in the fibrotic lungs of bleomycin-treated mice, thus decreasing the severity of the fibrotic condition [53]. Macrophages have also been shown to be critical for collagen degradation associated with liver fibrosis resolution, although the exact collagen-degrading mechanisms employed by the cells in this context is yet unknown [54].
Fig. 2.
Endocytic collagen receptors uPARAP/Endo180 and MR regulate collagens in disease. a uPARAP/Endo180 deficiency results in increased collagen accumulations after systemic CCl4 treatment in a mouse model of liver fibrosis. In this study, collagen levels in tissue sections from control livers (isolated from vehicle-injected mice) or fibrotic livers from wild-type mice or uPARAP/Endo180-deficient littermates injected with CCl4 in the peritoneum twice a week for 6 weeks were determined using Picrosirius Red staining and computer-assisted image analysis. Reproduced with permission from Wiley [17]. b uPARAP/Endo180 deficiency results in increased accumulation of collagen in a genetic model of mouse mammary tumors. Collagen levels were determined using immunohistochemical staining for collagen type I on tissue sections from mammary tumors isolated from female mice carrying the MMTV–PyMT transgene. Tumors were isolated from uPARAP-expressing mice (representative image in top left panel) or uPARAP/Endo180-deficient littermates (representative image in bottom left panel). Scale bar: 500 µm. The level of endogenous collagen inside tumors was determined using histomorphometric analysis (right panel). Reproduced with permission from Rockefeller University Press [16]. c MR drives the cellular uptake of collagens by TAMs and MR-deficiency results in increased collagen accumulation in a transplanted LLC tumor model. Fluorescently-labelled collagen was injected into syngeneic LLC tumors implanted in the subcutaneous space of C57BL/6 mice and the cellular uptake of collagen was visualized using confocal imaging of intact tumors isolated from wt mice (MRC1 + / + , upper left panel) and MR-deficient littermates (MRC1 −/−, upper right panel). The number of TAMs (F4/80 + CD11b +) with a high level of collagen uptake was quantified using flow cytometry-based analysis of single-cell suspensions generated by mechanical and enzymatical digestion of tumors (lower left panel). Intratumoral collagen levels in tumors from wt mice and MR-deficient littermates were determined using Picrosirius Red stainings of tumor tissue sections (lower center panels) and computer-assisted image analysis (lower right panel). Reproduced with permission from CellPress [10]
Cancer
Cancer invasion invariably includes degradation of ECM, including the breakdown of existing collagen scaffolds, and remodeling of de novo tumor-generated ECM. However, since this matrix differs markedly between different tissues and tumors, and since the collagen-degrading components differ between different cancer types, the mechanisms of collagen degradation are not uniform.
In the most common (carcinoma-type) cancers, the tumor cells themselves do not express endocytic collagen receptors, in accordance with the epithelial lineage of these cancers (Review: [31]). However, a strong upregulation of uPARAP/Endo180 occurs in tumor-associated non-malignant cells of carcinomas. This has been studied in detail in human mammary carcinoma, where a pronounced expression of uPARAP/Endo180 was observed in cancer-associated fibroblasts (CAFs) [55]. The upregulation of the receptor is functionally relevant, because studies in a genetic mouse mammary tumor model (the MMTV–PyMT model) showed increased accumulation of intra-tumor collagen in mice with inactivation of the uPARAP/Endo180 gene [16] (Fig. 2b). Furthermore, at least in some cases, the CAF-mediated uptake of tumor-associated collagen deposits appears to facilitate tumor progression, since the same study showed a delay in tumor growth in the uPARAP/Endo180-deficient mice. A strong stromal upregulation of uPARAP/Endo180 has also been observed in head-and-neck cancers [56] and several other carcinoma-type cancers (LH Engelholm, unpublished), although the contribution of uPARAP/Endo180 to collagen turnover and tumor progression in these cancers remains to be established.
In some less common cancer types, uPARAP/Endo180 may be expressed by the cancer cells themselves. This is the case in sarcomas including osteosarcoma [57], in the rare, and highly malignant, basal-like type of breast cancer [58] and in subsets of glioblastoma multiforme (GBM) [59]. In these cancers, uPARAP/Endo180 may prove useful as a pharmacological target, either through a functional blocking strategy [57] or as a mediator of targeted drug delivery, exploiting the capacity of uPARAP/Endo180 to efficiently internalize ligand [60].
Tumor-associated macrophages (TAMs) are also highly active in the uptake of collagen. This has been shown in mice carrying syngeneic Lewis lung carcinoma (LLC) cells, growing as subcutaneous tumors. In this model, TAMs were the dominant cell type in the uptake process, with MR being the responsible receptor and with the TAMs most likely originating from C–C chemokine receptor type 2 (CCR2)-positive monocytes [10]. As shown for uPARAP/Endo180 in the MMTV-PyMT mammary tumor model, ablation of MR in the LLC model also resulted in increased accumulation of intra-tumoral collagen [Fig. 2c, compare wild-type (MRC1 +/+) and MR-deficient mice (MRC1 −/−)].
Collagen and cultured immune cells
The above-described studies of uPARAP/Endo180 and MR in fibrosis and cancer have documented a key function of these receptors in collagen clearance in normal physiology and in several disease processes. The regulation of collagen levels by the two receptors has a profound impact on the cells imbedded in and interacting with the ECM, as shown by the enhanced fibrosis and impaired tumor progression associated with the ablation of uPARAP/Endo180 and MR. In the following sections, we will review the literature describing a functional relationship between collagen and immune cells of different origin, and we will discuss how receptors engaged in collagen uptake directly and indirectly may regulate immune responses in pathological conditions.
The major evidence showing that the ECM, and in particular collagen, modulates the behavior of immune cells originates from studies of cultured immune cells in various in vitro assays. In these assays, immune cells have typically been exposed to soluble forms of collagen, or grown on 2D collagen matrices or embedded in 3D collagen-based hydrogels. Although these model systems are highly simplified, they allow for ready manipulation of culture conditions and the effects of various types and densities of collagen can be evaluated. Already in 1976, Postlethwaite and co-workers discovered that soluble collagen fibrils and collagen fragmented into small peptides possessed the ability to attract cultured human monocytes, thereby providing some of the earliest evidence for collagen directly influencing immune cell function [61]. Since then, numerous examples of immune cell functions being affected by collagen have been reported. These studies include the demonstration that collagen type I can stimulate the differentiation of monocytes into macrophages [62] and induce the acquisition of an M2-like macrophage phenotype [63]. In support of this, we recently described that 3D cultured macrophages become more immunosuppressive when surrounded by a high collagen density compared to a low collagen density [64]. Furthermore, collagen has also been shown to stimulate the differentiation of monocytes into dendritic cells [65] and enhance dendritic cell activation [66]. The ability of other ECM molecules in tumors to interact with myeloid cells of the immune system and affect their functions has recently been reviewed [67].
The ability of dense 3D collagen matrices to impose physical constraints on T cells or in other ways guide or direct their movement has been documented [68–70], but additionally, we have also recently demonstrated that collagen can directly affect the activity of T cells [71]. Specifically, primary T cells that are 3D-cultured in collagen type I matrices of high density become less cytotoxically active compared to T cells cultured in a low collagen density. It is still unknown if this effect is related to the ability of T cells to sense changes in the stiffness of the surrounding matrix, although other groups have demonstrated that activation of the T-cell receptor can indeed be modulated by changes in substrate stiffness [72, 73].
Altogether, these in vitro studies indicate that collagen could promote the generation of an immunosuppressive tumor microenvironment by affecting myeloid cells as well as T cells.
Immune regulation by collagen in cancer
As a tumor develops, the gradual breakdown of local tissue ECM and the deposition of a new tumor-associated ECM generate a tumor-specific microenvironment with altered structural and cell-regulating properties [74, 75]. This tumor-specific ECM is typically very rich in collagen with a high degree of cross-linkage [76–80], which likely contributes to the increased tissue stiffness and density observed in different types of tumors.
Studies of breast cancer have so far provided the most insight into the impact of dense and stiff collagen structures on tumor progression, invasion, and metastasis. Breast tissue density, in large parts caused by high levels of deposited collagen, is a strong and independent risk factor for breast cancer development [81]. In addition, collagen density and the degree of collagen fiber alignment are associated with poor overall survival [82–84]. The role of collagen in breast cancer progression has also been studied in transgenic mice with manipulated collagen properties or with altered collagen degradation. In one such model, a knock-in mouse line expressing a mutated, collagenase-resistant form of collagen type I, known as the Col1a1tm1Jae mouse line [85] or collagen R/R mice, was combined with genetic mouse models of breast cancer [86, 87]. In another work, mice deficient for the collagenase, MMP-13, were studied in combination with a syngeneic, transplanted mammary cancer model [88], and in a third study, breast cancer models were combined with manipulation of collagen cross-linkage, either by inhibiting Lysyl oxidase (LOX) or by promotion of collagen cross-linkage by increased expression of LOX in the tumor stroma [77]. Collectively, these studies demonstrated that increased stromal collagen levels and cross-linkage in tumors are associated with enhanced tumor growth and metastasis. In many other human cancers, collagen density and stiffness has also been shown to correlate with invasion, disease aggressiveness, and clinical outcome [89–93].
The means by which increased collagen density and stiffness promote tumor progression in the above-mentioned studies are not well understood. But relevant to this open question, Pickup and co-workers have argued that all hallmarks of cancer are potentially influenced by tumor-associated changes to the ECM (review: [94]). Rooted in the increasing realization of the importance of immune regulation in cancer and the ability of collagen to influence cell behavior, it is tempting to speculate that increased collagen density and stiffness may be a pivotal mechanism for controlling immune cell phenotypes. In one likely scenario, an altered ECM may serve to ensure protection of tumors against immune destruction by cytotoxic T cells. In support of this hypothesis, studies of T cells in pancreatic and lung cancer suggest that dense stromal collagen structures impair T-cell migration and block these cells from coming into contact with tumor cells [70, 95]. This concept was also briefly reviewed by others in a context of immunotherapy efficacy, which may be negatively regulated by increased matrix density and stiffness [96].
Another immune cell type potentially influenced by the tumor-derived ECM is TAMs, with this question being particularly complicated in the light of the same cell type being directly involved in both the deposition and the degradation of collagen matrices in cancer [10, 97, 98]. In human luminal breast cancer lesions, increased deposition and stiffness of collagen has been reported in the invasive front. These differences in collagen correlated with an increased number of TAMs, when compared to the less collagen-rich core of the lesions or to non-invasive ductal carcinoma in situ [84]. In a study of the relationship between mammographic density, stromal collagen, and immune cells in prophylactically resected breast tissue from patients with high risk of cancer, regions of high mammographic density displayed increased levels of collagen and a reduced level of MR-positive macrophages when compared to regions with low mammographic density [99]. Although these studies point to somewhat opposite effects, they both suggest an intimate relationship between macrophage numbers or phenotype and collagen density and stiffness. However, they do not provide any immediate answer to the question of whether macrophage biology is mainly modulated by the matrix or vice versa.
Finally, neutrophils have also been reported to be affected by collagen density in tumors. García-Mendoza and co-workers combined the collagen R/R mice with a genetic breast cancer model and investigated the effects of depleting neutrophils using an antibody [100]. Neutrophil depletion did not affect breast tumors growing in wild-type mice, but both the burden of collagen-rich primary tumors and number of metastases were strongly reduced upon neutrophil depletion in the collagen R/R mice. These observations led the authors to speculate that the progression of tumors may be mechanistically different when collagen density increases [100].
Immune regulation by collagens in fibrotic disease
A large number of reports have addressed the basic mechanisms of fibrosis, the origin of collagen-producing myofibroblasts, and the events that lead to their activation, and excellent reviews are available on these topics (including recent reviews by [101, 102]). Many different types of immune cells have also been reported as instrumental in driving the pathogenesis of fibrosis, including macrophages, monocytes, dendritic cells, neutrophils, B cells, and T cells [103–108]. Subsets of T cells, dendritic cells, and macrophages have also been shown to protect against and help resolve fibrosis [54, 109–111], clearly illustrating the complicated biology of this type of disease. Despite the unquestionable contributions of immune cells to the development of fibrotic disease, very little is known about how immune cells themselves respond to the accumulation of collagen in affected tissues. The same applies to the role this might play in either the sustained pathogenesis of fibrosis and in fibrosis resolution.
Two studies that may shed some light on this matter include the original report of the generation of a mouse line expressing a collagenase-resistant mutated form of collagen type I [85] (see above) and a more recent report that characterizes a mouse line with a specific knock-out of the important collagenase, MMP-14/MT1-MMP, in dermal fibroblasts [6]. In these reports, the two mouse lines, which both have a reduced capacity for collagen degradation, present with an increased accumulation of collagen in the dermis in the absence of any external stimuli or tissue damage. Importantly, neither of these studies reported any signs of increased dermal presence of inflammatory cells, indicating that an elevated collagen level does not in its own right lead to the recruitment and activation of immune cells.
The above studies were done in an otherwise healthy setting. In the context of systemic sclerosis and various liver diseases, collagen, however, does appear to be able to mount an immune response, as evidenced by the detection of auto-antibodies against collagen type I and IV, among others, in patient sera [112–114]. Interestingly, the level of anti-collagen antibodies correlated with the degree of fibrosis [113]. Sawada et al. also reported that mononuclear cells from patients suffering from liver disease were sensitive to stimulation by collagens and that this property was not shared by cells from healthy subjects [114]. Providing additional evidence that the fibrotic ECM itself plays a role in maintaining and increasing a fibrotic response, decellularized lung ECM from idiopathic pulmonary fibrosis (IPF) patients stimulated the upregulation of genes encoding ECM proteins in primary fibroblasts, when compared to the effects of non-fibrotic lung ECM. This prompted the authors to suggest that a positive feedback loop which promotes further ECM deposition exists in IPF [115].
Based on the above-mentioned in vitro studies addressing the effect of collagens on immune cells and the studies of fibrosis discussed here, we speculate that a feedback mechanism in fibrosis may also encompass pro-fibrotic or anti-fibrotic immune cells that become sensitized and primed for collagen-stimulation. Such a mechanism may involve signaling or signaling-inhibitory molecules such as the collagen receptors leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1) and osteoclast-associated receptor (OSCAR), known to be expressed by immune cells. These receptors (see section below) could potentially be induced or upregulated during fibrosis, thereby enabling immune cells to directly respond to changes in the ECM. It follows that any such response of immune cells would be highly sensitive to those mechanisms that regulate collagen turnover in the ECM, including endocytic cellular uptake of collagens [17, 51, 52] or extracellular collagenolysis [116].
ECM-sensing collagen receptors expressed by immune cells
For changes in collagen level or structure to be perceived directly by immune cells, signalling receptors recognizing collagens are presumably required to be expressed on these cells. The majority of known mammalian collagen receptors, including collagen-binding integrins (e.g., β1-integrins), discoidin domain receptors (DDRs), and Glycoprotein VI, are mainly expressed on epithelial and mesenchymal cells, or on platelets (reviewed in [117]), although a few reports do suggest a role of DDRs in macrophage and T-cell movement in collagen matrices [118, 119]. Two collagen receptors with a more general immune-activating or immune-inhibitory potential have, however, been proposed. These are LAIR-1 and OSCAR, which we review in the following.
LAIR-1
The first immune regulatory receptor found to possess collagen-binding activity was the inhibitory LAIR-1 [120, 121]. LAIR-1 is broadly expressed by immune cells (Fig. 3) and interacts with a wide range of both ECM and cell surface-associated collagens [121–123]. The immune-inhibitory effects of LAIR-1 are exhibited through its two cytoplasmic Immune Receptor Tyrosine-based Inhibition Motifs (ITIMs) and have been thoroughly documented (reviewed in [124]). However, the potential of collagens to activate this receptor has been addressed in a limited number of in vitro studies only. These studies do include the recent demonstration that collagen type I fragments inhibit osteoclast formation through LAIR-1 in a negative feedback mechanism [125] and that soluble collagen type I and II blocks the activation of splenocytes and subsets of T cells via LAIR-1 [126].
Fig. 3.
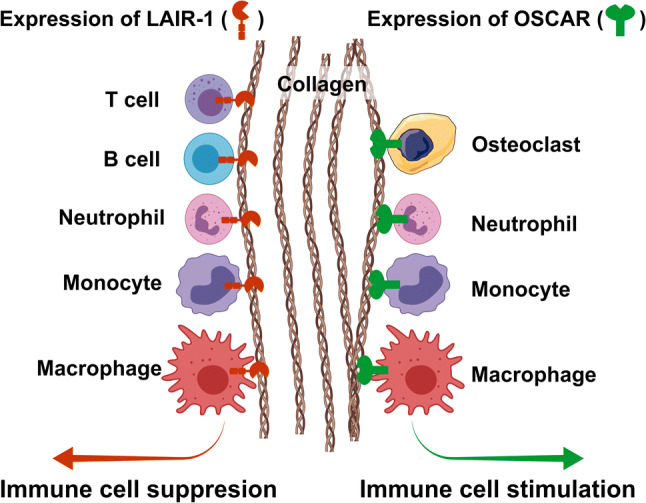
LAIR-1 and OSCAR and the regulation of immune cells by collagens. The collagen receptor LAIR-1 is expressed by the majority of immune cells, including T cells, B cells, neutrophils, macrophages, and monocytes. LAIR-1 generally functions as an immune-inhibitory collagen receptor, which, upon ligand binding, blocks the activation or differentiation of these cells. The collagen receptor OSCAR is expressed by human myeloid-derived immune cells, including osteoclasts, macrophages, monocytes, neutrophils, and dendritic cells (not shown). Opposite to LAIR-1, OSCAR functions as a stimulatory receptor, which activates immune cells through associations with FcRγ. In mice, the expression of OSCAR may be more limited and has so far only been demonstrated on osteoclasts
Two research groups have generated and characterized LAIR-1-deficient mice [127, 128] and neither group reported severe phenotypes in unchallenged mice. Smith and colleagues [128] focused on unravelling a role of LAIR-1 in platelet maturation, whereas Tang and colleagues [127] demonstrated that loss of LAIR-1 causes modest alterations in subsets of leukocytes in the spleen. However, in models of airway inflammation induced by respiratory syncytial virus infection or cigarette smoke exposure, a dramatic effect of LAIR-1 was found on neutrophil recruitment, with the receptor-deficient mice having highly elevated levels of these cells present in the alveolar space, resulting in more severe disease progression [129]. In models of arthritis, LAIR-1-deficient mice also developed a more severe disease, whereas mice in which LAIR-1 was activated using an antibody were protected from arthritis [126]. These animal studies confirm a potent inhibitory effect of LAIR-1 on immune cells, but, importantly, no studies have so far addressed the role of collagens in inducing altered immune cell phenotypes through LAIR-1 in vivo.
A few studies have recently addressed the expression of LAIR-1 in human cancers, including ovarian cancer [130], hepatocellular carcinoma [131], cervical cancer [132], and oral squamous cell carcinomas [133]. These studies revealed a common picture in which LAIR-1 is upregulated in the tumor stroma as compared to normal tissues, and also showed a positive correlation between LAIR-1 expression levels and the pathological grade of disease [131–133]. However, any potential correlations with collagen status and/or immune cell activity in tumors remain to be explored. Nonetheless, these studies in human cancers support that LAIR-1 may constitute a novel receptor that tumor cells can exploit to achieve immune evasion, a mechanism proposed as early as in 2010 [134]. With the successes of check-point inhibition-based cancer immune therapy in mind, exploration of the immune regulatory functions of LAIR-1 in vivo may prove highly valuable in future cancer immunology research, including investigations into the potential of blocking the collagen–LAIR-1 interactions as an anti-cancer treatment strategy.
OSCAR
Another immune receptor with collagen-binding activity is OSCAR. OSCAR was originally identified as a regulator of murine osteoclastogenesis [135], and to date, osteoclasts appear to be the main OSCAR-expressing cells in mice. In humans, the expression of OSCAR is broader and this receptor has been demonstrated on most myeloid cells [136, 137] (Fig. 3). In contrast to LAIR-1, OSCAR is an immune-activating receptor, which is enabled by its association with the Fc receptor common γ (FcRγ) [138, 139]. The discovery that OSCAR is a receptor for collagens [138] was correlated nicely with its function in osteoclastogenesis. Barrow and colleagues [138] also generated OSCAR-deficient mice and, using these mice, confirmed the osteoclast-related functions of the collagen receptor in vivo. The OSCAR null mice displayed a decrease in osteoclast numbers and size as well as a reduced bone erosion surface and increased trabecular bone volume, but only when OSCAR deficiency was combined with the loss of the DNAX-activating protein of 12 kDa (DAP12), an osteoclastogenesis-promoting adaptor protein, in OSCAR/DAP12 double-deficient mice. This suggests a large degree of redundancy within osteoclastogenesis. In two more recent studies, OSCAR-blocking antibodies were used to demonstrate that the collagen–OSCAR interaction stimulates a pro-inflammatory activation of human monocytes and monocyte-derived dendritic cells [65, 140]. Collagen type II was demonstrated to be especially potent in activating these immune cells. Combined with studies showing OSCAR expression in synovial tissue and fluids from patients suffering from rheumatoid arthritis [140, 141], and a positive correlation between OSCAR expression and disease activity [141], these observations have prompted the suggestion that OSCAR promotes ongoing joint disease [140].
Unfortunately, similar to LAIR-1, there is still a lack of studies addressing a role of OSCAR in regulating immune cells in conditions such as fibrotic disease and cancer. However, with the proposed opposite roles of LAIR-1 and OSCAR in immune regulation outlined above, the expression and activation of one or the other on immune cells may be a decisive factor determining if inflammation progresses or becomes resolved in pathological situations in which the ECM and collagens are key players (Fig. 3).
Interactions of collagen receptors with defense collagens of the innate immune system
Within the last 5–7 years, a handful of independent studies have shown that ECM-derived structural collagens share a number of receptors with defense collagens, a designation used for a group of soluble collagen-like proteins, which contain short triple-helical collagen domains and have functions in the innate immune system [142–145]. Collagen receptor/defense collagen couplings include the interactions between uPARAP, LAIR-1 and OSCAR with one or more of the defense collagens surfactant protein D (SP-D), mannose-binding lectin (MBL), and C1q. An interesting aspect of these interactions is highlighted by the defense collagens’ short collagen-like triple helix. Although this segment comprises 60 Gly-X–Y triplets or less in the defense collagens vs. typically more than 300 Gly-X-Y triplets in structural collagens, this is sufficient to harbour one or more binding sites for cell surface receptors with very different collagen-binding motifs. For example, uPARAP interacts with collagen via its fibronectin type-II domain [30], whereas LAIR-1 and OSCAR utilize distinct binding mechanisms involving extracellular Ig-like domains [146, 147]. However, regardless of differences in binding mechanism, each of these interactions between collagen receptors and defense collagens is likely a part of an immune regulatory pathway.
uPARAP/Endo180 and the cellular clearance of defense collagens
The majority of collagen subtypes, including collagens type I, IV, and V, are ligands for uPARAP/Endo180 and MR. Moreover, the two receptors appear to utilize very similar binding mechanisms in their interactions with structural collagens (see “Introduction”). Recently, we demonstrated that uPARAP/Endo180 is also active in the uptake and intracellular degradation of several defense collagens, including SP-D and MBL [142]. Surprisingly, this ability was not shared by MR. The uPARAP/Endo180-mediated uptake of defense collagens appears to be a mechanism by which activated fibroblasts, the main uPARAP/Endo180-expressing cells, can directly regulate immune responses. Following tissue injury, MBL and SP-D are recruited to extravascular sites, where they may either promote or dampen the resulting inflammation. For example, SP-D protects against lung injury caused by inflammation induced by bleomycin [148, 149], whereas MBL promotes injury induced by ischemia and reperfusion [150, 151]. Although the molecular mechanisms behind these functions of SP-D and MBL remain elusive, it is evident in both cases that their regulation and turnover are important for disease outcome. In this setting, the uPARAP/Endo180-mediated uptake and clearance of defense collagens constitutes a mechanism with the potential for both promoting and limiting inflammation [142].
LAIR-1 and OSCAR as receptors for defense collagens
The classical functions of defense collagens include the activation of complement and agglutination or opsonisation of pathogens. The discovery that defense collagens are ligands of collagen receptors expressed by immune cells naturally suggests a range of new potential functions of these components. C1q was the first defense collagen identified as a ligand for LAIR-1 [145], and not long after, SP-D was identified as a ligand of both LAIR-1 and OSCAR [143, 144].
In general, it seems that the effects of C1q and SP-D interacting with LAIR-1 and/or OSCAR are similar to the engagements of the two receptors with structural collagens (see above). C1q has been shown to bind LAIR-1 on the surface of human monocytes enriched from peripheral blood mononuclear cells and this interaction triggers LAIR-1 phosphorylation and a consequent immune suppression of these cells, which manifests as a reduced production of inflammatory cytokines and a block of differentiation [145, 152]. In a subsequent study, the same group suggested that the C1q–LAIR-1 interaction is part of a complicated inflammation-limiting mechanism by which monocytes can be differentiated to acquire a more M2-macrophage like phenotype [153].
The effects of SP-D interacting with LAIR-1 have, so far, been investigated using a human myeloid leukemia cell line. In these cells, SP-D blocked the release of reactive oxygen species via LAIR-1, thereby acting as an inflammation-limiting stimulus [143]. A more surprising result was reported regarding the outcome of the interaction of SP-D with OSCAR. This interaction was found to potently stimulate the release of TNFα by inflammatory monocytes [144]. The latter observation does not immediately conform to the conception of SP-D being an inflammation-limiting factor in respiratory diseases [148, 149].
Based on the limited number of studies addressing the relationships between defense collagens and the receptors OSCAR and LAIR-1, and the narrow range of experimental model systems used to study the connections so far, it is difficult to interpret their impact on immune cells and inflammatory processes in a general manner. However, with LAIR-1 expressed by the majority of immune cells and OSCAR by human myeloid cells, and with a whole battery of known defense collagens as potential ligands, there is clearly a potential for functional links in several human pathologies.
Conclusions
Collagen structures can undergo changes during disease progression as a result of unbalanced collagen synthesis and collagen degradation. In the latter process, several cellular uptake mechanisms play an important role. These collagen-related changes in the ECM have a strong potential for influencing the functions of resident cells imbedded in the matrix, and importantly, immune cells recruited as a result of injury. Immune cell movement can become physically restricted by increased collagen density, immune cells may become phenotypically activated by collagens or they may become inhibited. It is clear, however, that these processes are far from being completely understood. Future studies will provide answers to open questions such as how a dysregulated collagen homeostasis affects immune cell phenotype and gene expression at a more global level, if immune cell-associated collagen receptors become expressed in complex pathological conditions, and, not least, how interfering with the collagen-immune cell interactions might affect the clinical outcomes in diseases like fibrosis and cancer.
Acknowledgements
We thank Dr. Mary Jo Danton for critically reviewing this manuscript.
Funding
This study is supported by National Institute of Dental and Craniofacial Research (Intramural Research Program); Sundhed og Sygdom, Det Frie Forskningsråd (Grant Nos. 4092-00387B and DFF-4004-00340); Kræftens Bekæmpelse (Grant Nos. R90-A5989, R146-A9326-16-S2, R149-A9768-16-S47, R174-A11581-17-S52, R231-A13832, R222-A13103); Simon Fougner Hartmanns Familiefond; Region Hovedstadens forskningsfond; Novo Nordisk Fonden; Dansk Kræftforskningsfond.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Henrik J. Jürgensen, Email: hjj@finsenlab.dk
Daniel H. Madsen, Email: daniel.hargboel.madsen@regionh.dk
References
- 1.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields GB. Interstitial collagen catabolism. J Biol Chem. 2013;288(13):8785–8793. doi: 10.1074/jbc.R113.451211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rainero E. Extracellular matrix endocytosis in controlling matrix turnover and beyond: emerging roles in cancer. Biochem Soc Trans. 2016;44(5):1347–1354. doi: 10.1042/BST20160159. [DOI] [PubMed] [Google Scholar]
- 4.Amar S, Smith L, Fields GB. Fields GB (2017) Matrix metalloproteinase collagenolysis in health and disease. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):1940–1951. doi: 10.1016/j.bbamcr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake MT, Clarke BL, Oursler MJ, Khosla S. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. 2017;38(4):325–350. doi: 10.1210/er.2015-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zigrino P, Brinckmann J, Niehoff A, Lu Y, Giebeler N, Eckes B, Kadler KE, Mauch C. Fibroblast-derived MMP-14 regulates collagen homeostasis in adult skin. J Invest Dermatol. 2016;136(8):1575–1583. doi: 10.1016/j.jid.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016;52–54:363–383. doi: 10.1016/j.matbio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A. 2004;101(49):17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem. 2007;102(5):1130–1139. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- 10.Madsen DH, Jurgensen HJ, Siersbaek MS, Kuczek DE, Grey Cloud L, Liu S, Behrendt N, Grontved L, Weigert R, Bugge TH. Tumor-associated macrophages derived from circulating inflammatory monocytes degrade collagen through cellular uptake. Cell Rep. 2017;21(13):3662–3671. doi: 10.1016/j.celrep.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu El-Asrar AM, Mohammad G, Allegaert E, Ahmad A, Siddiquei MM, Alam K, Gikandi PW, De Hertogh G, Opdenakker G. Matrix metalloproteinase-14 is a biomarker of angiogenic activity in proliferative diabetic retinopathy. Mol Vis. 2018;24:394–406. [PMC free article] [PubMed] [Google Scholar]
- 13.Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Okada Y, Ikeda E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44(5):2163–2170. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol. 2011;31(9):e35–e44. doi: 10.1161/ATVBAHA.111.225623. [DOI] [PubMed] [Google Scholar]
- 15.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202(5):663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol. 2005;169(6):977–985. doi: 10.1083/jcb.200411153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen DH, Jurgensen HJ, Ingvarsen S, Melander MC, Vainer B, Egerod KL, Hald A, Rono B, Madsen CA, Bugge TH, Engelholm LH, Behrendt N. Endocytic collagen degradation: a novel mechanism involved in protection against liver fibrosis. J Pathol. 2012;227(1):94–105. doi: 10.1002/path.3981. [DOI] [PubMed] [Google Scholar]
- 18.Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28(4):229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- 19.Arora PD, Manolson MF, Downey GP, Sodek J, McCulloch CA. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J Biol Chem. 2000;275(45):35432–35441. doi: 10.1074/jbc.M003221200. [DOI] [PubMed] [Google Scholar]
- 20.Segal G, Lee W, Arora PD, McKee M, Downey G, McCulloch CA. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J Cell Sci. 2001;114(Pt 1):119–129. doi: 10.1242/jcs.114.1.119. [DOI] [PubMed] [Google Scholar]
- 21.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277(6):4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17(11):4812–4826. doi: 10.1091/mbc.e06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhide VM, Laschinger CA, Arora PD, Lee W, Hakkinen L, Larjava H, Sodek J, McCulloch CA. Collagen phagocytosis by fibroblasts is regulated by decorin. J Biol Chem. 2005;280(24):23103–23113. doi: 10.1074/jbc.M410060200. [DOI] [PubMed] [Google Scholar]
- 24.Shi F, Harman J, Fujiwara K, Sottile J. Collagen I matrix turnover is regulated by fibronectin polymerization. Am J Physiol Cell Physiol. 2010;298(5):C1265–1275. doi: 10.1152/ajpcell.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrendt N, Jensen ON, Engelholm LH, Mortz E, Mann M, Dano K. A urokinase receptor-associated protein with specific collagen binding properties. J Biol Chem. 2000;275(3):1993–2002. doi: 10.1074/jbc.275.3.1993. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh H, Yarwood H, Ashworth A, Isacke CM. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J Cell Sci. 2000;113(Pt 6):1021–1032. doi: 10.1242/jcs.113.6.1021. [DOI] [PubMed] [Google Scholar]
- 27.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572(2–3):364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 28.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176(11):6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 29.Napper CE, Drickamer K, Taylor ME. Collagen binding by the mannose receptor mediated through the fibronectin type II domain. Biochem J. 2006;395(3):579–586. doi: 10.1042/BJ20052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurgensen HJ, Johansson K, Madsen DH, Porse A, Melander MC, Sorensen KR, Nielsen C, Bugge TH, Behrendt N, Engelholm LH. Complex determinants in specific members of the mannose receptor family govern collagen endocytosis. J Biol Chem. 2014;289(11):7935–7947. doi: 10.1074/jbc.M113.512780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melander MC, Jurgensen HJ, Madsen DH, Engelholm LH, Behrendt N. The collagen receptor uPARAP/Endo180 in tissue degradation and cancer. Int J Oncol. 2015;47(4):1177–1188. doi: 10.3892/ijo.2015.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelholm LH, Nielsen BS, Netzel-Arnett S, Solberg H, Chen XD, Lopez Garcia JM, Lopez-Otin C, Young MF, Birkedal-Hansen H, Dano K, Lund LR, Behrendt N, Bugge TH. The urokinase plasminogen activator receptor-associated protein/endo180 is coexpressed with its interaction partners urokinase plasminogen activator receptor and matrix metalloprotease-13 during osteogenesis. Lab Invest. 2001;81(10):1403–1414. doi: 10.1038/labinvest.3780354. [DOI] [PubMed] [Google Scholar]
- 33.Abdelgawad ME, Soe K, Andersen TL, Merrild DM, Christiansen P, Kjaersgaard-Andersen P, Delaisse JM. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone. 2014;67:181–188. doi: 10.1016/j.bone.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Howard MJ, Isacke CM. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J Biol Chem. 2002;277(35):32320–32331. doi: 10.1074/jbc.M203631200. [DOI] [PubMed] [Google Scholar]
- 35.East L, McCarthy A, Wienke D, Sturge J, Ashworth A, Isacke CM. A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep. 2003;4(7):710–716. doi: 10.1038/sj.embor.embor882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelholm LH, List K, Netzel-Arnett S, Cukierman E, Mitola DJ, Aaronson H, Kjoller L, Larsen JK, Yamada KM, Strickland DK, Holmbeck K, Dano K, Birkedal-Hansen H, Behrendt N, Bugge TH. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J Cell Biol. 2003;160(7):1009–1015. doi: 10.1083/jcb.200211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjoller L, Engelholm LH, Hoyer-Hansen M, Dano K, Bugge TH, Behrendt N. uPARAP/endo180 directs lysosomal delivery and degradation of collagen IV. Exp Cell Res. 2004;293(1):106–116. doi: 10.1016/j.yexcr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Wienke D, MacFadyen JR, Isacke CM. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol Biol Cell. 2003;14(9):3592–3604. doi: 10.1091/mbc.e02-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mousavi SA, Sato M, Sporstol M, Smedsrod B, Berg T, Kojima N, Senoo H. Uptake of denatured collagen into hepatic stellate cells: evidence for the involvement of urokinase plasminogen activator receptor-associated protein/Endo180. Biochem J. 2005;387(Pt 1):39–46. doi: 10.1042/BJ20040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282(37):27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 41.Sprangers S, Behrendt N, Engelholm L, Cao Y, Everts V. Phagocytosis of collagen fibrils by fibroblasts in vivo is independent of the uPARAP/Endo180 receptor. J Cell Biochem. 2017;118(6):1590–1595. doi: 10.1002/jcb.25821. [DOI] [PubMed] [Google Scholar]
- 42.Madsen DH, Ingvarsen S, Jürgensen HJ, Melander MC, Kjøller L, Moyer A, Honoré C, Madsen CA, Garred P, Burgdorf S, Bugge TH, Behrendt N, Engelholm LH. The non-phagocytic route of collagen uptake. J Biol Chem. 2011;286(30):26996–27010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsen DH, Jurgensen HJ, Ingvarsen S, Melander MC, Albrechtsen R, Hald A, Holmbeck K, Bugge TH, Behrendt N, Engelholm LH. Differential actions of the endocytic collagen receptor uPARAP/Endo180 and the collagenase MMP-2 in bone homeostasis. PLoS ONE. 2013;8(8):e71261. doi: 10.1371/journal.pone.0071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27(18):6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fasquelle C, Sartelet A, Li W, Dive M, Tamma N, Michaux C, Druet T, Huijbers IJ, Isacke CM, Coppieters W, Georges M, Charlier C. Balancing selection of a frame-shift mutation in the MRC2 gene accounts for the outbreak of the Crooked Tail Syndrome in Belgian Blue Cattle. PLoS Genet. 2009;5(9):e1000666. doi: 10.1371/journal.pgen.1000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sartelet A, Klingbeil P, Franklin CK, Fasquelle C, Geron S, Isacke CM, Georges M, Charlier C. Allelic heterogeneity of Crooked Tail Syndrome: result of balancing selection? Anim Genet. 2012;43(5):604–607. doi: 10.1111/j.1365-2052.2011.02311.x. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Pomares L, Wienke D, Stillion R, McKenzie EJ, Arnold JN, Harris J, McGreal E, Sim RB, Isacke CM, Gordon S. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur J Immunol. 2006;36(5):1074–1082. doi: 10.1002/eji.200535685. [DOI] [PubMed] [Google Scholar]
- 48.Malovic I, Sorensen KK, Elvevold KH, Nedredal GI, Paulsen S, Erofeev AV, Smedsrod BH, McCourt PA. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology. 2007;45(6):1454–1461. doi: 10.1002/hep.21639. [DOI] [PubMed] [Google Scholar]
- 49.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, Burgdorf S, Engelholm LH, Behrendt N, Holmbeck K, Weigert R, Bugge TH. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202(6):951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jürgensen HJ, Silva LM, Krigslund O, van Putten SM, Madsen DH, Behrendt N, Engelholm LH, Bugge TH. CCL2/MCP-1 signaling drives extracellular matrix turnover by diverse macrophage subsets. Matrix Biol Plus. 2019 doi: 10.1016/j.mbplus.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Guisa JM, Cai X, Collins SJ, Yamaguchi I, Okamura DM, Bugge TH, Isacke CM, Emson CL, Turner SM, Shankland SJ, Eddy AA. Mannose receptor 2 attenuates renal fibrosis. J Am Soc Nephrol. 2012;23(2):236–251. doi: 10.1681/ASN.2011030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bundesmann MM, Wagner TE, Chow YH, Altemeier WA, Steinbach T, Schnapp LM. Role of urokinase plasminogen activator receptor-associated protein in mouse lung. Am J Respir Cell Mol Biol. 2012;46(2):233–239. doi: 10.1165/rcmb.2010-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119(12):3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(46):E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnack Nielsen B, Rank F, Engelholm LH, Holm A, Dano K, Behrendt N. Urokinase receptor-associated protein (uPARAP) is expressed in connection with malignant as well as benign lesions of the human breast and occurs in specific populations of stromal cells. Int J Cancer. 2002;98(5):656–664. doi: 10.1002/ijc.10227. [DOI] [PubMed] [Google Scholar]
- 56.Sulek J, Wagenaar-Miller RA, Shireman J, Molinolo A, Madsen DH, Engelholm LH, Behrendt N, Bugge TH. Increased expression of the collagen internalization receptor uPARAP/Endo180 in the stroma of head and neck cancer. J Histochem Cytochem. 2007;55(4):347–353. doi: 10.1369/jhc.6A7133.2006. [DOI] [PubMed] [Google Scholar]
- 57.Engelholm LH, Melander MC, Hald A, Persson M, Madsen DH, Jurgensen HJ, Johansson K, Nielsen C, Norregaard KS, Ingvarsen SZ, Kjaer A, Trovik CS, Laerum OD, Bugge TH, Eide J, Behrendt N. Targeting a novel bone degradation pathway in primary bone cancer by inactivation of the collagen receptor uPARAP/Endo180. J Pathol. 2016;238(1):120–133. doi: 10.1002/path.4661. [DOI] [PubMed] [Google Scholar]
- 58.Wienke D, Davies GC, Johnson DA, Sturge J, Lambros MB, Savage K, Elsheikh SE, Green AR, Ellis IO, Robertson D, Reis-Filho JS, Isacke CM. The collagen receptor Endo180 (CD280) is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res. 2007;67(21):10230–10240. doi: 10.1158/0008-5472.CAN-06-3496. [DOI] [PubMed] [Google Scholar]
- 59.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS ONE. 2010;5(3):e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen CF, van Putten SM, Lund IK, Melander MC, Norregaard KS, Jurgensen HJ, Reckzeh K, Christensen KR, Ingvarsen SZ, Gardsvoll H, Jensen KE, Hamerlik P, Engelholm LH, Behrendt N. The collagen receptor uPARAP/Endo180 as a novel target for antibody-drug conjugate mediated treatment of mesenchymal and leukemic cancers. Oncotarget. 2017;8(27):44605–44624. doi: 10.18632/oncotarget.17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Postlethwaite AE, Kang AH. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wesley RB, 2nd, Meng X, Godin D, Galis ZS. Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler Thromb Vasc Biol. 1998;18(3):432–440. doi: 10.1161/01.atv.18.3.432. [DOI] [PubMed] [Google Scholar]
- 63.Stahl M, Schupp J, Jager B, Schmid M, Zissel G, Muller-Quernheim J, Prasse A. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE. 2013;8(11):e81382. doi: 10.1371/journal.pone.0081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsen AMH, Kuczek DE, Kalvisa A, Siersbæk MS, Thorseth M-L, Carretta M, Grøntved L, Vang O, Madsen DH. Collagen density modulates the immunosuppressive functions of tumor-associated macrophages. bioRxiv. 2019 doi: 10.1101/513986. [DOI] [PubMed] [Google Scholar]
- 65.Schultz HS, Nitze LM, Zeuthen LH, Keller P, Gruhler A, Pass J, Chen J, Guo L, Fleetwood AJ, Hamilton JA, Berchtold MW, Panina S. Collagen induces maturation of human monocyte-derived dendritic cells by signaling through osteoclast-associated receptor. J Immunol. 2015;194(7):3169–3179. doi: 10.4049/jimmunol.1402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poudel B, Yoon DS, Lee JH, Lee YM, Kim DK. Collagen I enhances functional activities of human monocyte-derived dendritic cells via discoidin domain receptor 2. Cell Immunol. 2012;278(1–2):95–102. doi: 10.1016/j.cellimm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Sangaletti S, Chiodoni C, Tripodo C, Colombo MP. Common extracellular matrix regulation of myeloid cell activity in the bone marrow and tumor microenvironments. Cancer Immunol Immunother. 2017;66(8):1059–1067. doi: 10.1007/s00262-017-2014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pruitt HC, Lewis D, Ciccaglione M, Connor S, Smith Q, Hickey JW, Schneck JP, Gerecht S. Collagen fiber structure guides 3D motility of cytotoxic T lymphocytes. Matrix Biol. 2019 doi: 10.1016/j.matbio.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201(7):1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20(13):3422–3433. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 71.Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbaek MS, Simoes AMC, Roslind A, Engelholm LH, Noessner E, Donia M, Svane IM, Straten PT, Grontved L, Madsen DH. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7(1):68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Kam LC, Milone MC. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189(3):1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng Y, Reinherz EL, Lang MJ. alphabeta T cell receptor mechanosensing forces out serial engagement. Trends Immunol. 2018;39(8):596–609. doi: 10.1016/j.it.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2011;3(1):a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Terajima M, Yang Y, Sun L, Ahn YH, Pankova D, Puperi DS, Watanabe T, Kim MP, Blackmon SH, Rodriguez J, Liu H, Behrens C, Wistuba II, Minelli R, Scott KL, Sanchez-Adams J, Guilak F, Pati D, Thilaganathan N, Burns AR, Creighton CJ, Martinez ED, Zal T, Grande-Allen KJ, Yamauchi M, Kurie JM. Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J Clin Invest. 2015;125(3):1147–1162. doi: 10.1172/JCI74725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(21):7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 80.Ng MR, Brugge JS. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16(6):455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 82.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bredfeldt JS, Liu Y, Conklin MW, Keely PJ, Mackie TR, Eliceiri KW. Automated quantification of aligned collagen for human breast carcinoma prognosis. J Pathol Inform. 2014;5(1):28. doi: 10.4103/2153-3539.139707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7(10):1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130(1):227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barcus CE, O'Leary KA, Brockman JL, Rugowski DE, Liu Y, Garcia N, Yu M, Keely PJ, Eliceiri KW, Schuler LA. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017;19(1):9. doi: 10.1186/s13058-017-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry SW, Schueckler JM, Burke K, Arcuri GL, Brown EB. Stromal matrix metalloprotease-13 knockout alters Collagen I structure at the tumor-host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC Cancer. 2013;13:411. doi: 10.1186/1471-2407-13-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vellinga TT, den Uil S, Rinkes IH, Marvin D, Ponsioen B, Alvarez-Varela A, Fatrai S, Scheele C, Zwijnenburg DA, Snippert H, Vermeulen L, Medema JP, Stockmann HB, Koster J, Fijneman RJ, de Rooij J, Kranenburg O. Collagen-rich stroma in aggressive colon tumors induces mesenchymal gene expression and tumor cell invasion. Oncogene. 2016;35(40):5263–5271. doi: 10.1038/onc.2016.60. [DOI] [PubMed] [Google Scholar]
- 90.Zou X, Feng B, Dong T, Yan G, Tan B, Shen H, Huang A, Zhang X, Zhang M, Yang P, Zheng M, Zhang Y. Up-regulation of type I collagen during tumorigenesis of colorectal cancer revealed by quantitative proteomic analysis. J Proteom. 2013;94:473–485. doi: 10.1016/j.jprot.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 91.Jolly LA, Novitskiy S, Owens P, Massoll N, Cheng N, Fang W, Moses HL, Franco AT. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 2016;76(7):1804–1813. doi: 10.1158/0008-5472.CAN-15-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohno S, Tachibana M, Fujii T, Ueda S, Kubota H, Nagasue N. Role of stromal collagen in immunomodulation and prognosis of advanced gastric carcinoma. Int J Cancer. 2002;97(6):770–774. doi: 10.1002/ijc.10144. [DOI] [PubMed] [Google Scholar]
- 93.Li HX, Zheng JH, Fan HX, Li HP, Gao ZX, Chen D. Expression of alphavbeta6 integrin and collagen fibre in oral squamous cell carcinoma: association with clinical outcomes and prognostic implications. J Oral Pathol Med. 2013;42(7):547–556. doi: 10.1111/jop.12044. [DOI] [PubMed] [Google Scholar]
- 94.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang H, Hegde S, DeNardo DG. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother. 2017;66(8):1037–1048. doi: 10.1007/s00262-017-2003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger T, Sagi I, Varol C. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213(11):2315–2331. doi: 10.1084/jem.20151193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Varol C. Tumorigenic interplay between macrophages and collagenous matrix in the tumor microenvironment. Methods Mol Biol. 2019;1944:203–220. doi: 10.1007/978-1-4939-9095-5_15. [DOI] [PubMed] [Google Scholar]
- 99.Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L, Brown KA, Magenau A, Allam AH, McGhee E, Timpson P, Henderson MA, Thompson EW, Britt K. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia-Mendoza MG, Inman DR, Ponik SM, Jeffery JJ, Sheerar DS, Van Doorn RR, Keely PJ. Neutrophils drive accelerated tumor progression in the collagen-dense mammary tumor microenvironment. Breast Cancer Res. 2016;18(1):49. doi: 10.1186/s13058-016-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Pakshir P, Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68–69:81–93. doi: 10.1016/j.matbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia H, Yacoub TJ, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raker V, Haub J, Stojanovic A, Cerwenka A, Schuppan D, Steinbrink K. Early inflammatory players in cutanous fibrosis. J Dermatol Sci. 2017;87(3):228–235. doi: 10.1016/j.jdermsci.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, Koteliansky V, Hochman PS, Ibraghimov A. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115(11):3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ, 2nd, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126(1):50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 108.Lebleu VS, Sugimoto H, Miller CA, Gattone VH, 2nd, Kalluri R. Lymphocytes are dispensable for glomerulonephritis but required for renal interstitial fibrosis in matrix defect-induced Alport renal disease. Lab Invest. 2008;88(3):284–292. doi: 10.1038/labinvest.3700715. [DOI] [PubMed] [Google Scholar]
- 109.Artsen AM, Rytel M, Liang R, King GE, Meyn L, Abramowitch SD, Moalli PA. Mesh induced fibrosis: the protective role of T regulatory cells. Acta Biomater. 2019;96:203–210. doi: 10.1016/j.actbio.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, Friedman SL, Merad M, Aloman C. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55(1):244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pociask DA, Chen K, Choi SM, Oury TD, Steele C, Kolls JK. gammadelta T cells attenuate bleomycin-induced fibrosis through the production of CXCL10. Am J Pathol. 2011;178(3):1167–1176. doi: 10.1016/j.ajpath.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mackel AM, DeLustro F, DeLustro B, Fudenberg HH, LeRoy EC. Immune response to connective tissue components of the basement membrane. Connect Tissue Res. 1982;10(3–4):333–343. doi: 10.3109/03008208209008058. [DOI] [PubMed] [Google Scholar]
- 113.Borg BB, Seetharam A, Subramanian V, Basha HI, Lisker-Melman M, Korenblat K, Anderson CD, Shenoy S, Chapman WC, Crippin JS, Mohanakumar T. Immune response to extracellular matrix collagen in chronic hepatitis C-induced liver fibrosis. Liver Transpl. 2011;17(7):814–823. doi: 10.1002/lt.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sawada H, Suou T, Hirayama C. Cellular sensitivity to collagen in liver disease. Clin Exp Immunol. 1985;59(2):364–370. [PMC free article] [PubMed] [Google Scholar]
- 115.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 118.Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circ Res. 2009;105(11):1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- 119.Hachehouche LN, Chetoui N, Aoudjit F. Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Mol Immunol. 2010;47(9):1866–1869. doi: 10.1016/j.molimm.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 120.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 121.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, Meyaard L. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203(6):1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lebbink RJ, de Ruiter T, Kaptijn GJ, Bihan DG, Jansen CA, Lenting PJ, Meyaard L. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int Immunol. 2007;19(8):1011–1019. doi: 10.1093/intimm/dxm071. [DOI] [PubMed] [Google Scholar]
- 123.Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. 2011;49(1–2):402–406. doi: 10.1016/j.molimm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 124.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83(4):799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 125.Boraschi-Diaz I, Mort JS, Bromme D, Senis YA, Mazharian A, Komarova SV. Collagen type I degradation fragments act through the collagen receptor LAIR-1 to provide a negative feedback for osteoclast formation. Bone. 2018;117:23–30. doi: 10.1016/j.bone.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Kim S, Easterling ER, Price LC, Smith SL, Coligan JE, Park JE, Brand DD, Rosloniec EF, Stuart JM, Kang AH, Myers LK. The role of leukocyte-associated ig-like receptor-1 in suppressing collagen-induced arthritis. J Immunol. 2017;199(8):2692–2700. doi: 10.4049/jimmunol.1700271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang X, Tian L, Esteso G, Choi SC, Barrow AD, Colonna M, Borrego F, Coligan JE. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J Immunol. 2012;188(2):548–558. doi: 10.4049/jimmunol.1102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith CW, Thomas SG, Raslan Z, Patel P, Byrne M, Lordkipanidze M, Bem D, Meyaard L, Senis YA, Watson SP, Mazharian A. Mice lacking the inhibitory collagen receptor LAIR-1 exhibit a mild thrombocytosis and hyperactive platelets. Arterioscler Thromb Vasc Biol. 2017;37(5):823–835. doi: 10.1161/ATVBAHA.117.309253. [DOI] [PubMed] [Google Scholar]
- 129.Kumawat K, Geerdink RJ, Hennus MP, Roda MA, van Ark I, Leusink-Muis T, Folkerts G, van Oort-Jansen A, Mazharian A, Watson SP, Coenjaerts FE, Bont L, Meyaard L. LAIR-1 limits neutrophilic airway inflammation. Front Immunol. 2019;10:842. doi: 10.3389/fimmu.2019.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flies DB, Higuchi T, Harris JC, Jha V, Gimotty PA, Adams SF. Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103(+) dendritic cells in late-stage ovarian cancer. Oncoimmunology. 2016;5(8):e1185583. doi: 10.1080/2162402X.2016.1185583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu X, Zhang L, Zhou J, Liu L, Fu Q, Fu A, Feng X, Xin R, Liu H, Gao Y, Xue J. Clinicopathologic significance of LAIR-1 expression in hepatocellular carcinoma. Curr Probl Cancer. 2019;43(1):18–26. doi: 10.1016/j.currproblcancer.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Zhang X, Miao F, Cao Y, Xue J, Cao Q, Zhang X. Clinical significance of leukocyte-associated immunoglobulin-like receptor-1 expression in human cervical cancer. Exp Ther Med. 2016;12(6):3699–3705. doi: 10.3892/etm.2016.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang LL, Zhang MJ, Wu L, Mao L, Chen L, Yu GT, Deng WW, Zhang WF, Liu B, Sun WK, Sun ZJ. LAIR-1 overexpression and correlation with advanced pathological grade and immune suppressive status in oral squamous cell carcinoma. Head Neck. 2019;41(4):1080–1086. doi: 10.1002/hed.25539. [DOI] [PubMed] [Google Scholar]
- 134.Meyaard L. LAIR and collagens in immune regulation. Immunol Lett. 2010;128(1):26–28. doi: 10.1016/j.imlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 135.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195(2):201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Merck E, Gaillard C, Gorman DM, Montero-Julian F, Durand I, Zurawski SM, Menetrier-Caux C, Carra G, Lebecque S, Trinchieri G, Bates EE. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004;104(5):1386–1395. doi: 10.1182/blood-2004-03-0850. [DOI] [PubMed] [Google Scholar]
- 137.Merck E, Gaillard C, Scuiller M, Scapini P, Cassatella MA, Trinchieri G, Bates EE. Ligation of the FcR gamma chain-associated human osteoclast-associated receptor enhances the proinflammatory responses of human monocytes and neutrophils. J Immunol. 2006;176(5):3149–3156. doi: 10.4049/jimmunol.176.5.3149. [DOI] [PubMed] [Google Scholar]
- 138.Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, Delaisse JM, Takayanagi H, Lorenzo J, Colonna M, Farndale RW, Choi Y, Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest. 2011;121(9):3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Merck E, de Saint-Vis B, Scuiller M, Gaillard C, Caux C, Trinchieri G, Bates EE. Fc receptor gamma-chain activation via hOSCAR induces survival and maturation of dendritic cells and modulates toll-like receptor responses. Blood. 2005;105(9):3623–3632. doi: 10.1182/blood-2004-07-2809. [DOI] [PubMed] [Google Scholar]
- 140.Schultz HS, Guo L, Keller P, Fleetwood AJ, Sun M, Guo W, Ma C, Hamilton JA, Bjorkdahl O, Berchtold MW, Panina S. OSCAR-collagen signaling in monocytes plays a proinflammatory role and may contribute to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2016;46(4):952–963. doi: 10.1002/eji.201545986. [DOI] [PubMed] [Google Scholar]
- 141.Herman S, Muller RB, Kronke G, Zwerina J, Redlich K, Hueber AJ, Gelse H, Neumann E, Muller-Ladner U, Schett G. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):3041–3050. doi: 10.1002/art.23943. [DOI] [PubMed] [Google Scholar]
- 142.Jurgensen HJ, Norregaard KS, Sibree MM, Santoni-Rugiu E, Madsen DH, Wassilew K, Krustrup D, Garred P, Bugge TH, Engelholm LH, Behrendt N. Immune regulation by fibroblasts in tissue injury depends on uPARAP-mediated uptake of collectins. J Cell Biol. 2019;218(1):333–349. doi: 10.1083/jcb.201802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olde Nordkamp MJ, van Eijk M, Urbanus RT, Bont L, Haagsman HP, Meyaard L. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J Leukoc Biol. 2014;96(1):105–111. doi: 10.1189/jlb.3AB0213-092RR. [DOI] [PubMed] [Google Scholar]
- 144.Barrow AD, Palarasah Y, Bugatti M, Holehouse AS, Byers DE, Holtzman MJ, Vermi W, Skjodt K, Crouch E, Colonna M. OSCAR is a receptor for surfactant protein D that activates TNF-alpha release from human CCR2+ inflammatory monocytes. J Immunol. 2015;194(7):3317–3326. doi: 10.4049/jimmunol.1402289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci U S A. 2012;109(46):E3160–3167. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brondijk TH, de Ruiter T, Ballering J, Wienk H, Lebbink RJ, van Ingen H, Boelens R, Farndale RW, Meyaard L, Huizinga EG. Crystal structure and collagen-binding site of immune inhibitory receptor LAIR-1: unexpected implications for collagen binding by platelet receptor GPVI. Blood. 2010;115(7):1364–1373. doi: 10.1182/blood-2009-10-246322. [DOI] [PubMed] [Google Scholar]
- 147.Zhou L, Hinerman JM, Blaszczyk M, Miller JL, Conrady DG, Barrow AD, Chirgadze DY, Bihan D, Farndale RW, Herr AB. Structural basis for collagen recognition by the immune receptor OSCAR. Blood. 2016;127(5):529–537. doi: 10.1182/blood-2015-08-667055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Casey J, Kaplan J, Atochina-Vasserman EN, Gow AJ, Kadire H, Tomer Y, Fisher JH, Hawgood S, Savani RC, Beers MF. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am J Respir Crit Care Med. 2005;172(7):869–877. doi: 10.1164/rccm.200505-767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med. 2012;185(5):525–536. doi: 10.1164/rccm.201103-0561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van der Pol P, Schlagwein N, van Gijlswijk DJ, Berger SP, Roos A, Bajema IM, de Boer HC, de Fijter JW, Stahl GL, Daha MR, van Kooten C. Mannan-binding lectin mediates renal ischemia/reperfusion injury independent of complement activation. Am J Transplant. 2012;12(4):877–887. doi: 10.1111/j.1600-6143.2011.03887.x. [DOI] [PubMed] [Google Scholar]
- 151.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175(1):541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 152.Son M, Diamond B. C1q-mediated repression of human monocytes is regulated by leukocyte-associated Ig-like receptor 1 (LAIR-1) Mol Med. 2015;20:559–568. doi: 10.2119/molmed.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Son M, Porat A, He M, Suurmond J, Santiago-Schwarz F, Andersson U, Coleman TR, Volpe BT, Tracey KJ, Al-Abed Y, Diamond B. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood. 2016;128(18):2218–2228. doi: 10.1182/blood-2016-05-719757. [DOI] [PMC free article] [PubMed] [Google Scholar]