Abstract
Concomitant with advances in research regarding the role of miRNAs in sustaining carcinogenesis, major concerns about their delivery options for anticancer therapies have been raised. The answer to this problem may come from the world of nanoparticles such as liposomes, exosomes, polymers, dendrimers, mesoporous silica nanoparticles, quantum dots and metal-based nanoparticles which have been proved as versatile and valuable vehicles for many biomolecules including miRNAs. In another train of thoughts, the general scheme of miRNA modulation consists in inhibition of oncomiRNA expression and restoration of tumor suppressor ones. The codelivery of two miRNAs or miRNAs in combination with chemotherapeutics or small molecules was also proposed. The present review presents the latest advancements in miRNA delivery based on nanoparticle-related strategies.
Keywords: miRNA therapy, Nanodelivery, Nanoparticles, Cancer
Introduction
The human genome encodes for a great variety of transcripts. However, the majority of RNAs do not interact with the ribosome and do not possess protein coding capacity, therefore they are included in the category of non-coding RNAs (ncRNAs) [1].
MicroRNAs (miRNAs, miRs) are part of the ncRNA category and have a sequence length of 19–25 nucleotides [2–4]. Generally, they interact with the messenger RNAs (mRNAs) of various genes and repress their translation. The interaction is mediated by a small miRNA region of 3 nucleotides called the seed region. Because of this characteristic, miRNAs are able to target a great number of mRNAs, where the same mRNA can be targeted by multiple miRNAs [5–7].
The high range of targeted mRNAs can constitute an advantage for cancer therapy, because it has a simultaneous effect on multiple cellular pathways [8–10]. There are generally two types of miRNAs with relevance for the oncological pathologies: tumor suppressor miRNAs and oncomiRNAs (oncomiRs). The tumor suppressor ones inhibit the development of malignant entities and are usually down-regulated in cancer. The oncomiRs support the malignant phenotype and are overexpressed [11, 12]. However, these parameters are often cancer specific, where one miRNA can be overexpressed in a specific type of malignancy and inhibited in other [13]. The general approach for a miRNA-based anticancer therapy relies on the inhibition of oncomiRs and/or the up-regulation of tumor suppressor ones. This is done with the help of exogenous anti-miRNAs in the case of oncogenic miRNAs and mimic sequences for the tumor suppressor miRNAs [14–16].
However, miRNA-based therapies pose a number of challenges. Such examples consist in the naked miRNAs that are usually degraded by enzymes presented in the blood stream or are not able to pass through the extracellular matrix. Additionally, miRNAs can also activate the Toll-like receptors and induce inflammation [17]. To overcome these challenges, the conjugation of miRNAs with a cholesterol molecule at the passenger strand has been proposed. However, this method is quite inefficient, since it requires a high concentration of RNA molecules [18].
Most RNAs are internalized through endocytosis. Inside the cell, an endosome is formed through the budding of the plasma membrane. The endosome first fuses with a lysosome, then with the Golgi apparatus, and its content is completely digested. If the miRNA molecule does not manage to escape the endosome in due time, it will no longer be able to exert its therapeutic activity [19, 20]. The main strategy for endosomal escape consists in the induced disruption of the endosomal membrane [21, 22]. Moreover, the presence of ribonucleases in cytoplasm makes miRNA delivery even more difficult [23]. However, several chemical modifications were developed such as phosphorothioate containing oligonucleotides, methyl-oligonucleotides, methoxyethyl-oligonucleotides [24], locked nucleic acid oligonucleotides [25], peptide nucleic acids [26] or fluorine derivatives [27].
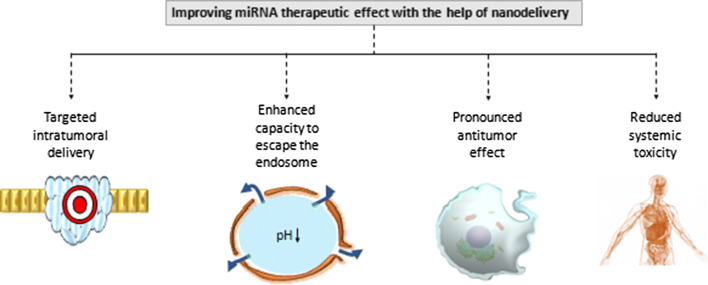
The nanoparticle-based delivery of miRNAs is highly efficient and has a number of advantages in comparison with the delivery of naked chemically modified transcripts. Some of the advantages include the following: a greater intratumoral accumulation, enhanced capacity to escape the endosome, greater therapeutic effect and reduced systemic toxicity (Fig. 1).
Fig. 1.
Enhanced therapeutic effect of miRNA through nanoparticles. First, miRNA molecules accumulate in a higher concentration inside the tumor and bypass to a greater degree the normal cells. Second, the endosomal escape is greatly facilitated. Third, because of higher intra-cytoplasmic accumulation a more pronounced antitumor effect is achieved. Last, due to enhanced targeted effect a reduced systemic toxicity and hence decreased side effects are observed
In this review, we analyze the up-to-date nanoparticle-based transporters of miRNAs used in cancer therapy. For this, we take into consideration a variety of nanodelivery systems, from the most biocompatible nanoparticles, such as liposomes, exosomes, dendrimers and polymeric nanoparticles to the metal-based ones which possess the great advantage of unique optical and/or magnetic properties and hence, can be simultaneously combined with other therapies such as plasmonic or magnetic hyperthermia. Last, newly developed nanodelivery systems are presented including quantum dots (QDs) and mesoporous silica nanoparticles (MSN).
General consideration about nanoparticle design
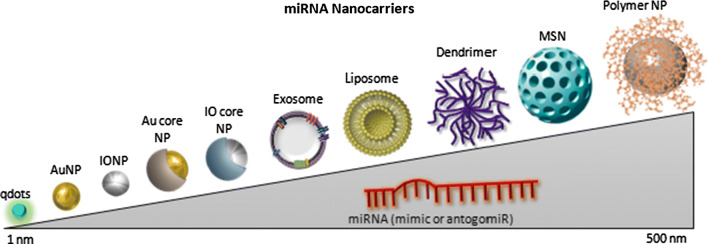
The NP size is one of the most important characteristic when taking into consideration their delivery to biological organisms (Fig. 2). If the NP is too large, it will not be eliminated by the renal tubules and hence, it can cause major organ failure. On the other hand, the NP should be able to avoid being internalized by the resident macrophages and to escape from the blood circulation through the vessel pores. At the tumor site, the particle must overcome differences in pressure between the intra- and the extratumoral environment [28, 29].
Fig. 2.
Representation of the main miRNA nanocarriers according to their size. The nanoparticles used for therapeutic miRNA delivery have a size range of 1–500 nm (although generally recommended maximum size is up to 100 nm). In the order of size range the qdots are the smallest ones, followed by metallic nanoparticles (AuNPs and IONPs), core–shell NPs (Au or IO core), liposomes, dendrimers, MSNs and polymeric NPs
The nanoparticles are generally employed in cancer due to their capability to specifically target cancer cells and, therefore, enhance the accumulation of the drug at the tumor site. The enhanced permeability and retention (EPR) effect is generally a characteristic of the tumor microenvironment and refers to the hyper-permeability of the blood network in cancer spots and also to the lack of functional lymphatics at the tumor mass [30]. These two characteristics are allowing nanoparticles of certain dimensions to enter within the malignant mass due to increased intercellular spaces between endothelial cells and also to stay there for a longer time in the lack of a proper lymphatic system. The spaces between the endothelial cells of normal vessels measures less than 10 nm, which facilitates the entry of any nanoparticle bigger than this size in the tumor microenvironment [31, 32].
The charge of the NPs is another aspect to consider. If the NP surface is cationic, it will cause membrane disruption of all the encountered cells. On the other hand, an anionic NP would be degradated inside the cell, probably in a lysosomal dependent manner [33]. In general, the citotoxicity of the nanoparticles is a crucial aspect to be taken in consideration; the current studies in oncology are mainly focused on the therapeutic effect and to a lesser extent on the biocompatibility of these vehicles. Moreover, there is a lack of standardized methods/models for the evaluation of such effects and also an the inability of the several employed protocols to relieve the entire toxicity spectrum [34]. The aspect of toxicity will be discussed in the following chapters for each type of described nanomaterial.
The shape, surface chemistry, composition and size of the nanoparticles are also greatly influencing the modality by which their internalization by the cells is made. Generally, there are three main mechanisms for nanoparticle entry into the intracellular space: direct diffusion or disruption, endocytosis—fluid-phase or receptor-mediated and via ion channel or transporter proteins. Direct diffusion or disruption of the lipid bilayer involves the permeabilisation of the membrane by the nanoparticle itself; in this case, there are numerous variables that need to be taken into consideration in order to assess the potential of particle entry: charge, hydrophobicity, size, shape and composition [35]. A widely discussed mechanism and also the one that is thought to be the most common for nanoparticle entry in the cell consists in endocytosis. This process comprises the wrapping of the lipid bilayer around the particle that will be internalized, followed by their budding and pinching off toward the formation of endocytic vesicle. There are different types of endocytosis depending on the molecules involved in the process and also on the cell type; the main mechanisms involve the following: phagocytosis, clathrin-mediated endocytosis, clathrin/caveolae-independent endocytosis, caveolin-mediated endocytosis, and macropinocytosis [35]. Depending on the first steps within the internalization, endocytosis can be classify into fluid phase or receptor mediated; in the case of the first one, the cargo is located in the extracellular fluid, also close to a forming vesicle and is subsequently captured by the structure and internalized inside the vesicle that will finally be endocytosed. For the case of receptor-mediated mechanism, the internalization of the cargo inside the vesicle is done via a component of the cell membrane that binds the nanoparticle and also undergoes vesicle trapping [36]. Lastly, one of the rarest forms of internalization of NPs is mediated by ion channel and transporter proteins that are present in the plasma membrane of the targeted cells and function as translocaters of extracellular cargos inside the intracellular space. However, the high degree of selectivity and small pore sizes are suggesting that this mechanism is rarely encountered during NP entering into cell [36]. Detailed analysis of the NP internalization and about the interaction within the environment/tumor microenvironment before trapping into the targeted cells are presented by Behzadi et al. [35] and Zhao and Stenzel [37].
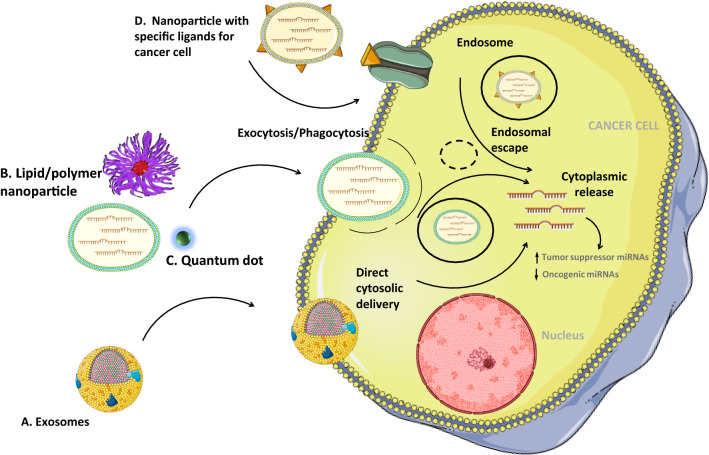
Figure 3 is presenting the general mechanisms by which a nanovehicle can enter into tumor cells and release its cargo.
Fig. 3.
General mechanisms by which a nanovehicle can enter the tumor cells and release its cargo. Nanovehicles can enter within cancer cells through membranes fusion (a), endocytosis/phagocytosis (b, c) or by receptor-mediated binding. Moreover, the content of the nanovehicles can be directly released into the cytoplasm as in the case of exosomes, whereas in other cases, the vehicle has to undergo endosomal pathway. Either the pathway or the final point consists in the cytoplasmic release of miRNAs with further effects upon the endogenous level of non-coding RNAs—upregulation of tumor suppressor miRNAs and downregulation of the oncogenic ones
Lipid-based nanoparticles
Liposomes
Liposomes as nanodelivery systems have attracted the research field due to their easy synthesis and functionalization method. In addition, they are highly stable, have a high loading efficiency and low cytotoxicity. The cationic lipids can be easily loaded with miRNAs due to chemical interactions between their positive charge and the negative charge of the transcripts [38]. However, the in vivo delivery of liposomes as neutrally charged lipids is recommended due to the toxicity of positively charged molecules [39]. Also, liposomes offer the possibility of combining miRNA delivery with different chemotherapeutic drugs, resulting in a synergic and improved therapeutic effect [38].
The typical formulation of liposomes implies the formation of amphiphilic phospholipid bilayers that entrap an aqueous core; the therapeutic cargo can be included inside these liposomes or can be entrapped on their surface (coated liposomes). In the case of liposomal delivery of miRNA/siRNA (siRNAs are exogenous sequences that can block the translation of specific genes [40]), the encapsulation strategies are inspired by Lipofectamine transfection. Therefore, some protocols suggest incubating miRNA molecules with synthesized liposomes, and based on their different charges, the electrostatic interactions will take place [41, 42]. On the other hand, there are some protocols that recommend to encapsulate the miRNA during liposome synthesis [43–45]. The determination of the encapsulation rate is quite difficult, but it was proved to be higher in the second strategy.
One of the most advanced studies for miRNA delivery in liposomes is represented by MRX34 formulation, the first-in-human, phase I study involving interventional miRNA-based strategies in cancer (upregulation of miR-34a in patients diagnosed with advanced solid tumors). The patients were enrolled based on their refractory status to standard treatment and received intravenous doses of liposomal miR-34a mimic for 3 weeks. The composition of the liposomal content was based on amphoteric lipids that are cationic in the process of acidic liposome formation to facilitate efficient encapsulation of the miR-34a mimic that is negatively charged, and anionic in neutral pH in vivo, with the aim of reducing the possibility of particle aggregation and the electrostatic adherence between the liposomes and the endothelial cells. Although some improvements were seen in the evolution of the oncological clinical parameters, the installation of adverse effects (AEs) associated with the infusion of MRX34 impaired the immediate continuation of the next clinical trial phase. The AEs were not specifically attributed to the liposomal carrier or to the miR-34a sequence [46]. However, Tolcher et al. used the same delivery vehicle for Bcl-2 targeting via ssDNA drug—PNT2258 with minimal side effects. This excludes to a certain point (although the studies cannot be directly compared) the toxicity of the liposomal components [47].
In lung cancer cells, let-7a mimic sequences were successfully delivered with the help of a liposome, resulting in reduced proliferation, invasion and increased apoptosis. Specifically, the sequence was found as downregulated in this malignancy, where experimental upregulation could destabilize the cancer cell. To increase the specificity of the delivery, the liposomal nanoparticles were conjugated with ephrin-A1, due to the increased expression of the corresponding receptor—ephrin type-A receptor 2 (EphA2)—in cancer, including non-small-cell lung cancer (NSCLC); moreover, ephrin-A1 reduces the migration and proliferation of malignant cell. miR–ephrin-A1–LNP successfully increased the expression of let-7a, while the expression levels of H-RAS, N-RAS and K-RAS were decreased. These effects were superior to free let-7a miRNA delivery, especially due to increased accumulation of the sequence in cancer cells [42]. In hepatocellular carcinoma (HCC) cells, delivery of anti-miR-122 ferried in a pH-sensitive cationic liposome (YSK05-MEND) resulted in a more pronounced antitumoral activity in liver cancer cells, when compared to the administration of the same miRNA with Lipofectamine; this was due to a more efficient endosomal escape in the case of cationic liposomes. In vivo treatment with AMO122 resulted in increased clearance via kidneys due to the molecular weight of the therapeutic molecule and its minimal concentration within the liver. The same cargo administrated inside YSK05-MEND accumulated to a higher extent in the liver, together with upregulating the miR-122 target genes. The effect persisted for 2 weeks, hence, demonstrating the feasibility of the nanovehicle [48]. In the same pathology and using the same miRNA therapeutic, another group established a negatively charged liposomal cargo targeted toward HCC cells expressing the transferin (Tf) receptor. The modification of the liposomes toward targeting of Tf increased the efficiency of the delivery by 15-fold compared with the non-modified liposomes, together with upregulation of PTEN, P27kip1, and TIMP3. Successful administration was also done in vivo through intravenous injection in HepG2 tumor xenografted mice [49].
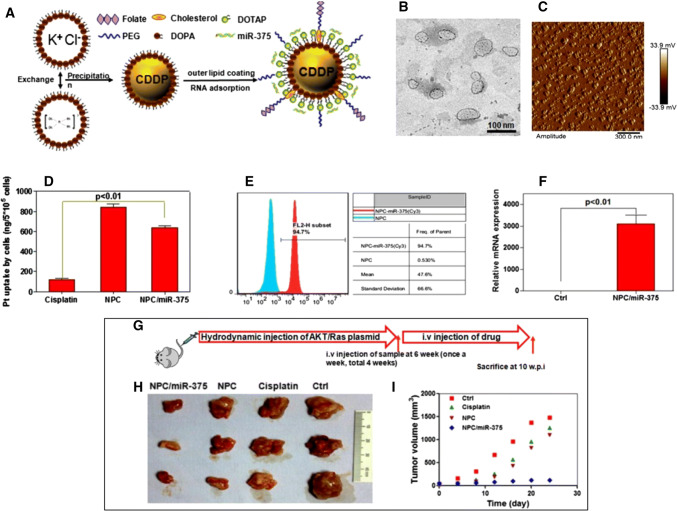
As reminded, the lipid-based nanodelivery of miRNAs can imply the codelivery with a chemotherapeutic agent. Cisplatin-coated liposomes loaded with miR-375 in HCC were used for the enhancement of cisplatin therapy and impairment of drug resistance. The nanoparticles were prepared by mixing two reverse microemulsions with KCl solution and also a soluble cis-diaminedihydroplatinum (II) covered with a cationic lipid layer, after which miR-375 was introduced into the nanoparticles already conjugated with cisplatin. In vitro studies showed that this type of co-delivery resulted in efficiently escaped lysosome degradation and increased apoptosis rate together with cell cycle arrest. Moreover, a significant effect was also observed in Akt/Ras-induced primary HCC mouse model that was treated in multiple series with effects upon tumor growth and relapse (Fig. 4) [50].
Fig. 4.
Modulation of HCC progression via cisplatin-coated liposomes co-loaded with miR-375. a Schematic representation of the nanocomplex NPC/miR-375 b TEM and c AFM characterization of the nanocomplex d Uptake of cisplatin in HepG2 cells measured by ICP-MS; miR-375 level in HepG2 cells measured by e FACS and f RT-qPCR. g Schematic illustration of the in vivo experimental model h Excised tumors and i volume from control and treated mice.
Reprinted (adapted) with permission from Yang et al. [50]
A similar approach in concept was also used for HCC treatment, although with miR-101-3p combined with doxorubicin (DOX). Doxorubicin liposomes and miR-101-3p were mixed in a 200:1 ration, in water, followed by 2–3 min vortexing and 30 min RT incubation. The combined therapy impaired invasion, colony formation and migration in vitro; in this case too, the xenograft tumors had a smaller volume [51].
Codelivery strategies can also be applied in the case of other miRNAs: miR34a mimic and miR-21 anti-sense sequence are encapsulated within targeted cationic liposomes that are directed toward the epidermal growth factor receptor (EGFR), usually overexpressed by cancer cells. This approach was used for treatment of glioblastoma multiforme that is a highly aggressive and resistant tumor. Under this strategy, a reduction in cell viability and invasion with decrease of pro-proliferation gene markers and upregulation of the pro-aproptotic genes were observed. In vivo systemic injection resulted in accumulation of the complex inside the tumor with effects upon the volume of the malignant mass. According to the group knowledge, this study was the first that used multiple miRNA administration inside a targeted cationic liposome [52].
The main studies that used liposomes as delivery systems for therapeutic miRNAs are included in Table 1.
Table 1.
Liposome-based miRNA delivery used their therapeutic effect
| Cell lines | Loaded therapeutic agent | Biological effect | Molecular changes | References |
|---|---|---|---|---|
| Hepa1c1c7 | antimiR-122 | Improved intratumor accumulation | Up-regulation of ALDOA, BCKDK, NDRG3 | [48] |
| HUVECs,26 NL-17 | miR-499 | Anti-angiogenic, improved intratumor accumulation, small tumor volume | Down-regulation of FZD8, CnAα,VEGFA | [53] |
| CRL-2081, CRL-5830, A549 | let-7a | Increased cellular uptake, reduced proliferation and invasion, small tumor volume | Down-regulation of H-RAS, N-RAS and K-RAS | [42] |
| HepG2 | antimiR-221 | No effect on cell proliferation, increased apoptosis, cell cycle arrest | Up-regulation of PTEN, P27kip1, TIMP3 | [49] |
| HepG2, Hep3B | miR-375 cisplatin | Decreased viability, increased apoptosis and cell cycle arrest, small tumor size, accumulation in the liver, kidney and tumor | NA | [50] |
| A549 | antimiR-21 paclitaxel | Decreased viability (paclitaxel), decreased invasion/migration, small tumor size | Up-regulation of PTEN, PDCD4, RECK, TIMP3, ANKRD46 DDAH1 | [54] |
| CNE1, CNE2, SUNE1, H0NE1, 5-8F, 6-10B C666-1 | miR-124-3p | Decreased proliferation, invasion/migration, no effect on apoptosis | Down-regulation of STAT3, CCND2, MMP-2 | [55] |
| SMMC-7721, Huh7 and HepG2 cell | miR-101 | Decreased invasion/migration colony formation, increased apoptosis, small tumor size | Down-regulation of NLK, EZH2, MCL-1, STMN1, RAB5A | [51] |
| MCF-7, ZR-75-1, L929 | antimiR-191 | Decreased viability, increased cell death, decreased tumorigenicity and migration | NA | [56] |
Exosomes
In order to obtain a targeted delivery of miRNA, researchers have proposed the use of natural delivery systems under the name of exosomes. These small vesicles of 40–100 nm are generated through endocytosis and are considered functional carriers of information between multiple cell types. They are found in various body fluids, such as blood, lymph, saliva or urine [57–60]. The exosomes have a specific pattern of proteins on their surface, which facilitates the identification of the targeted cell [3, 61].
The potential of exosomes as diagnostic or therapeutic tools was abundantly analyzed in various cancers, such as breast cancer [62] or hepatocellular carcinoma [63].
When used as drug delivery systems, exosomes present a number of advantages and disadvantages. On one hand, they can avoid the endosomal-lysosomal system and deliver their cargo directly into the cytoplasm. They are also highly stable when circulating through the body fluids. On the other hand, they can give an immunogenic reaction, especially due to the presence of specific ligands on their surface [64]. As for example, exosome-like vesicles were less effective for the delivery of RNA interference sequences, in comparison with liposomes. Specifically, the main limitation consisted in the lack of reproductivity of the drug loading system and also in the efficiency of these strategies; the attachment of siRNA to the surface of exosome-like vesicles (ELVs) via cholesterol anchor failed to deliver the cargo in dendritic and lung epithelial cell lines despite the successful uptake of exosomes. Also, surface-bound cholesterol-conjugated siRNA liposomes inhibited the expression of the targeted mRNA under the same experimental conditions [65].
However, exosomes were successfully used for the delivery of miRNA in glioblastoma (pathology difficult to approach due to the blood–brain-barrier); bone marrow-derived mesenchymal stem cells (MSCs) were cultured ex vivo and transduced with miR-124 lentiviral vector, miRNA that was further found in the exosomes harvested from the media. Systemic administration of Exo-miR124 in mice with intracranial GSC267 increased significantly the survival rate, with 50% of the animals living long term [66]. Despite the therapeutic role of Exo-miR124, the study also showed that MSCs can be used as “natural biofactories” for modified exosomes. However, the selection of the modified exosome-producing cells should be done within the context of a specific pathology and constantly considering the communication between “factory” and targeted cells, the rate of the exosome production, also the capacity of the exosomes to incorporate exogenous sequences.
For enhanced specificity, the surface of exosomes can be modified to selectively target specific cells. This strategy was applied for specific targeting of HepG2 cells with exosomes loaded with miR-26a, sequence downregulated in liver cancer. The sequences for CD63 and Apo-A1-binding peptide were introduced into a pEGFP-N2 vector that was further used for tranfection of HEK 293T cells with the purpose of inducing the secretion of engineered exosomes. These exosomes were further mixed with Cy5-labeled miR-26a and loaded with the non-coding cargo via electroporation. Alteration of exosomal membrane increased the specificity for HepG2 cells; also, the release of exosomal cargo upregulated the miR-26a levels in the liver cancer cells with effects upon inhibition of cell migration and proliferation. In triple negative breast cancer (TNBC), delivery of miR-134 through miR-134-enriched EVs produced in Hs578Ts(i)8 cells after transfection with miR-34 resulted in reduced migration and invasion of the recipient cells and decreased Hsp90 level. However, there were no significant changes in proliferation or cisplatin-induced apoptosis like in the case of direct transfection with miR-134. miR-134-enriched EVs increased, however, the sensitivity of TNBC cells to anti-Hsp90 drugs [67]. The immune system cells can be also exploited for exosome production. In a recent study, B cell-derived exosomes (from non-stimulated B cells, stimulated ones and also from macrophages) were loaded with miR-155 by electroporation under optimized voltage and they were further treated with RNase H for elimination of non-loaded miR-155 sequences. It was further shown that the exosomes from the stimulated B cells lack almost completely endogenous miR-155 sequences and can be used as successful delivery vehicles for this miRNA; moreover, these exosomes (once loaded with miR-155 mimic) can deliver the non-coding RNA into primary mouse hepatocytes with a 700-fold increase compared to control counterparts. Similar results were obtained in miR-155 knockout mice that received intravenous doses of miRNA-155 loaded exosomes with further effects on the upregulation of the miRNA in the isolated hepatocytes. Strikingly, this elevation was observed 10 min after the intravenous injection, demonstrating the efficiency of exosomes as delivery vehicles for miRNA therapeutics. The same group previously demonstrated that overexpression of miR-155 in RAW 264.7 macrophages induced by LPS is resulting in an increased production of TNFα. Using the same exosomes isolated from the activated B cells, miR-155 inhibitors were delivered into macrophages with effects upon reduction of miR-155 endogenous level, increase in SOCS1 expression and downregulation of TNFα protein. Additionally, no significant cytotoxicity was observed. Comparing with other tested methods: transfection reagents (FuGENE® HD and HiPerFect), the exosomes were superior in delivering the miRNA inhibitor and also exerted a higher percentage of cytotoxicity [68].
The main studies that used exosomes as a delivery platform for therapeutic miRNAs are included in Table 2.
Table 2.
Exosome-based miRNA delivery and their therapeutic effects
| Cell lines | Loaded therapeutic agent | Biological effect | Molecular changes | References |
|---|---|---|---|---|
| HEK293T, HepG2 | miR-26a | Inhibition of cell migration and proliferation, inhibited cell cycle progression | Down-regulation of CCNE2, CDK6, CCND2 | [69] |
| GSC267, GSC20, GSC6-27, GSC8-11, and GSC2-14 | miR-124a | Reduction in viability and clonogenicity, no long term evidence of tumor | Down-regulation of FOX A2 | [66] |
| BGC-823 | miR-21 inhibitor | Increased apoptosis | Down-regulation of PDCD4 | [70] |
| Hs578T | miR-134 | Reduced proliferation, migration and invasion | Down-regulation of BCL-2, STAT5B, HSP90, | [67] |
| MCF-7 | let-7a | Smaller tumor size | Down-regulation of RAS, HMGA2 | [71] |
Polymer-based nanoparticles
Polymeric nanoparticles
Polymeric NPs present a significant number of advantages due to their physicochemical properties. Their general size ranges between 100 and 500 nm. There are four main types of production methods: solvent evaporation, emulsification–solvent diffusion, solvent displacement and monomer polymerization. The preparation of nanoparticles for drug delivery depends mainly on the method used for cargo loading, resulting in amphiphilic core/shell (polymeric micelles), capsules (polymeric nanoparticles or polymeric nanoconjugates), or hyperbranched macromolecules of nanometer dimensions (dendrimers) [72]. miRNA polymeric encapsulation is similar to liposomal encapsulation [73, 74]. The most preferred polymers are those that ensure biocompatibility and biodegradability. In addition, the class of stimuli responsive polymers are highly recommended for their ability to release the cargo under the action of physical or chemical stimuli [75]. The level of control over cargo release depends also on the 3D structure of the polymer [76].
Some of the advantages of using polymeric NPs in delivery applications are the ease of their synthesis, their reduced costs, biodegradability, increased biocompatibility, prolonged circulation time, sustained release, fewer side effects, availability of different functionalization strategies, water solubility and high accumulation at tumor site [76–78]. Also, cationic polymers are thought to be more stable than cationic lipids and, therefore, associated with prolonged protection in cellular trafficking [78].
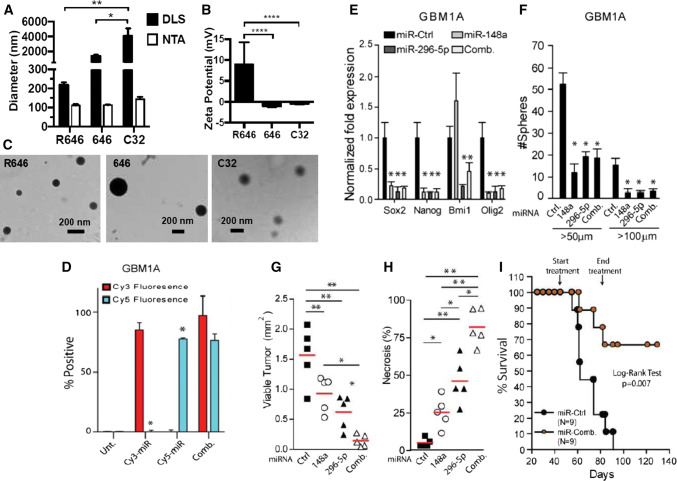
In miRNA delivery applications, the bioreducible poly(beta-amino ester) (PBAE) nanoparticles conjugated with both miR-148a and miR-296-5p showed accelerated and increased delivery inside the targeted cells, reduced toxicity and the ability to escape from endosomes when used to target the stem-like phenotype of GBM neurospheres. Moreover, the efficiency of these polymeric nanoparticles (R646 nano-miRs) was found to be increased compared to the commercial products like RNAiMax and far superior to the PEI and Lipofectamine 3000 transfection reagents. The success of the co-delivery of the two miRNAs was obtained by prior mixing of the two anionic miRNAs before the addition of the cationic polymer for the establishment of the nanoparticle self-assembly. The concentration of both miRNAs was set at 60 nM each, resulting in a final co-delivery of 120 nM of miRNA cargo. Following the accumulation in the two patient-derived neurospheres, the level of miR-148a increased by 16-fold and of miR-296-5p by 30-fold, respectively, with a constant expression up to 12 days. The increase in the two non-coding sequences inhibited the ability of sphere-formation, together with the decrease of Sox2, Nanog, Bmi1, Olig2, Dnmt1 and Hmga1 genes. Similar effects were obtained in vivo in GBM xenografts, contributing to the long-term survival of the mouse models [79]. Figure 5 presents part of the original data, adapted from the reminded research strategy.
Fig. 5.
Polymer R646 forms nanoparticles with miRNA and effectively releases miRNAs in the tumor environment. a R646 nano-miRs have significantly lower hydrodynamic diameter and b higher zeta potential c morphology of the nanoparticles by TEM d flow citometry for delivery of Cy3-labeled miRNA, Cy5-labeled miRNA and combination between the two into GBM1A neurospheres e stem cell markers in GBM1A neurospheres after transfection with nano-miRs f neurosphere numbers (> 100 μm diameter) after transfection with nano-miRs g viable tumor tissue (h) and necrotic tumor tissue in mice treated with nano-miRs i percent of mice survival in days treated or not with nano‐miRs.
Reprinted (adapted) with the permission from Lopez-Bertoni et al. [79]
For glioblastoma treatment, the codelivery of antagomiR-21 and antagomiR-10b (10 pmol each) conjugated with cRGD-tagged PEG-PLGA nanoparticles increased the sensitivity of U87MG and Ln229 cells to temozolomide (TMZ) in low doses. Co-inhibition of the tro-miRNAs before the administration of TMZ resulted in decreased viability and cell cycle arrest at G2/M phase. The in vitro effect was also mirrored in animal models with a reduction of tumor volume and increased sensitivity even to the lowest dose of TMZ. Moreover, the cRGD-functionalized PLGA polymer (preparation: PLGA-PEG: PLGA-cRGD: PLGA-Cy7.5–80:10:10) was more efficient than the non-targeted one (PLGA-PEG: PLGA-Cy7.5–90:10) by approximately threefold difference 24 h after treatment; however, no significant differences were shown after 48 h [80].
The main studies that used polymeric NPs as delivery systems for therapeutic miRNAs are included in Table 3.
Table 3.
Polymer- and dendrimer-based miRNAs delivery and their therapeutic effects
| Cell lines | Loaded therapeutic agent | Polymer type/name | Biological effect | Molecular changes | Model of study | References |
|---|---|---|---|---|---|---|
| GBM1A or GBM1B | miR-148a and miR-296-5 | Bioreducible poly(β-amino ester) nanoparticles (R646 nano-miRs) | Reduced the stem-like characteristics | Down-regulation of SOC2, NANOG, BMI, OLIG2 | In vitro: human GBM cells and orthotopic In vivo: human GBM xenografts | [79] |
| MDA-MB-231 | antagomiR-221, miR-205 mimic | RNA-triple-helix hydrogel nanoconjugates | Decreased invasion and tumorigenicity, smaller tumor size, no systemic toxicity | NA | In vivo: orthotopic TNBC mouse model | [94] |
| U251 | miR-7 | poly(amido amine) (FA/PAMAM/miR-7) | Decreased proliferation and invasion, increased apoptosis, smaller tumor size | Down-regulation of EGFR, PI3 K, AKT2 |
In vitro: human glioma cells In vivo: brain-glioma mouse model |
[95] |
| A549 | miR-34a | S6 aptamer-conjugated dendrimer (PAM-Ap/pMiR-34a) | Decreased viability, increased apoptosis, migration, invasion | Up-regulation of p53, BCL-2 (only mRNA) | In vitro: human NSCLC cells | [100] |
| Huh7 | antimiR122 PNA | Porous silicon nanoparticles (PSNPs)—polymer nanocomposites | Improved bioavailability | NA |
In vitro: Human hepatocellular carcinoma cells In vivo: C57BL/6 J mice for evaluation of liver related parameters |
[101] |
| U87MG (HTB-14) and Ln229 (CRL2611) | antagomiR-21 and antagomiR-10b | cRGD-tagged PEG-PLGA nanoparticles | Increased apoptosis, impaired cell cycle progression |
Down-regulation of PTEN (mRNA) Up-regulation of PDCD4, PTEN (protein), CASP3 l |
In vitro: human glioblastoma multiforme (GBM) cells In vivo: human GBM cell xenografts mice model |
[80] |
Dendrimers
A special type of polymeric NPs is represented by dendrimers. The formation of dendrimers begins with a core molecule, which branches out through a polymeric reaction; the synthesis of these branches can be convergent or divergent. Due to the versatility of the included monomers, the dendrimers are polyvalent and can bind a great variety of molecules: from drugs belonging to different classes to various nucleic acids [81, 82].
There are three types of dendrimers: Poly propylene imine (PPI) dendrimers, Polyamidoamine (PAMAM) dendrimers and Poly-l-lysine (PLL) dendrimers. These NPs can easily penetrate through the tumor vascular network and have a high intratumoral accumulation [83]. However, dendrimers present in vivo toxicity that is mainly caused by their positively charged surface which determines the formation of nanoholes in the cell membrane leading to membrane disruption. As follows, cytotoxicity upon normal cell and various hematological disorders were reported in animal models [84, 85]. Two methods were developed to overcome this challenge: synthesis of biocompatible dendrimers and the masking of the positive charge branches [84]. The first one implies the synthesis of dedrimers with a core and branching units that are biodegradable—usually this synthesis involves the usage of metabolic products under the form of monomers, resulted from different biological pathways [86]. Such examples consist in polyether dendrimers, polyester dendrimers, polyether imine dendrimers, polyether—copolyester (PEPE) dendrimers, phosphate dendrimers, citric acid dendrimers, melamine dendrimers, peptide dendrimers and triazine dendrimers [84]. The second method applied for reducing the dendrimer toxicity consists in the masking of the positive surface charge and it takes place through surface engineering. Specifically, the nanoparticles are conjugated through covalent or non-covalent binding with different moieties that finally protects the amine groups and decreases the overall cytotoxicity [87, 88]. This coating usually comes with additional benefits related to improved pharmacokinetic, drug encapsulation and cell targeting, better solubility, increased transfection efficiency, and higher stability [89–93]. The coating can take place in various forms: PEGylation, carbohydrate engineering, acetylation, half generation- or anionic, amino acid or peptide conjugation, drug and DNA/gene conjugation, antibody functionalization, tuftsin, folate conjugation, miscellaneous and others [84]. Extensive details about the toxicity of dendrimers and strategies for overcoming this difficulty are discussed in the article by Jain et al. [84].
Examples of miRNA delivery via dendrimer-mediated delivery include tests on triple negative breast cancer (TNBC), a form of breast cancer not responsive to conventional hormonal therapy. Through the assemble of a RNA-triple-helix to polyamidoamine (PAMAM) G5 dendrimer (triplex nanoparticles) and further mixing with dextran aldehyde that allows the formation of an adhesive hydrogel, the authors obtained a vehicle that allows the controlled and local release of two miRNAs (miR-205 sense and antagomiR-221). The in vitro uptake efficiency of the RNA-dendrimer was measured by flow citometry, which showed that this therapeutic formulation was able to penetrate almost 100% of the cells in a strong and uniform manner. The internalization started 3 h after addition of RNA-dendrimers in the media and achieved a maximum value 48 h after treatment. The effects consisted in smaller tumor size, lower invasion capacity and decreased tumorigenic potential in an orthotopic breast cancer mouse model. Moreover, the RNA-triple-helix hydrogel scaffolds comprising both miRNA modulators determined a 90% reduction in tumor volume, while each miRNA separately was associated with 50% inhibition rate. No systemic toxicity was observed and no accumulation of the triplex nanoparticles in major organs, with the exception of some traces of the nanoparticle in the intestine during the 13 days treatment. Interestingly, the release kinetics of the two miRNAs was different as demonstrated by the point of maximum fluorescence: 24 h for miR-205 mimic and 48 h for miR-221 inhibitor [94]. The PAMAM dendrimer was also conjugated with miR-7 and tested on a glioblastoma cell line. It resulted in inhibition of proliferation, invasion, migration and tumorigenesis, while the EGFR, PI3 K and AKT2 expression levels were significantly decreased [95].
Aptamers are short sequences of single-stranded DNA or RNA selected by SELEX process (systematic evolution of ligands by exponential enrichment) that are able to fold in three-dimensional conformations and bind protein targets [96, 97]. The advantage of aptamers besides antibodies consists in low immunogenicity, decreased toxicity, fast tissue penetration and high stability [98, 99]. In non-small cell lung cancer (NSCLC), a PAMAM-PEG-aptamer connection was created by conjugation of an S6 aptamer selected against A549 lung cancer cell line with a PAMAM dendrimer, complex that was further conjugated with miR-34a (PAM-Ap/pMiR-34a). The nanoparticle complex caused decreased viability, migration and invasion when applied in vitro; at the molecular level, the expression of p53 was stimulated and the level of BCL-2 decreased at both transcript and protein level [100]. The main studies that used dendrimers as a delivery system for therapeutic miRNAs are included in Table 3.
Mesoporous silica nanoparticles
Mesoporous silica nanoparticles (MSNs) are a type of NPs that are beginning to gain increased popularity among the delivery platforms for cancer therapy. They are biodegradable, their pores offer a larger loading surface, and they can offer a controlled drug release [102, 103]. Specifically, the size of the pore, which can be adjusted to each particular load, is critical for the release behavior of the drugs loaded to MSNs. This parameter was investigated in the case of itraconazole by evaluating the release performance at four SBA-15 MSNs with pore size varying from 4.5 to 9.0 nm. Functional studies showed that the increase of the pore size from 4.5 to 6.4 nm significantly improved the release of itraconazole, while a further increase to 7.9 and 9.0 nm revealed modest improvement in the release profile. The presented data show that there is a critical size of the pores where the drug diffusion can be adapted [104]. According to the Higuchi equation, the pore connectivity/geometry is also an essential parameter in controlling the diffusion of a drug from MSNs. Heikkilä et al. [105] compared the ibuprofen dissolution rate according to the materials and pore conformations of three types of MSNs: MCM-41 and SBA-15—uniform and unidirectional structure; thermally carbonized porous silicon (TCPSi)—2D mesoporous material with different pore sizes ranging from 2 to 30 nm and finally a silica material, TUD-1 (Technische Universiteit Delft), with networks of mesopores from 2.5 to 20 nm connected in a 3D structure similar to a sponge and also with a high accessibility. The loading of the drug was highly connected to the total pore volume, with SBA-15 having the highest loading capacity and TUD-1 the lowest; however, the greatest release of ibuprofen was obtained with TUD-1 material due to its reachable conformation (3D network), followed by SBA-15 with the most sizeable pores. Another parameter for drug release is the surface area. However, no significant correlation was highlighted in a study evaluating the release of aceclofenac once this parameter reached a limiting value [106]. Also, different surface functionalization of MSNs can improve the drug dissolution rate [107–109]. Another advantage of MSNs consists in low immunogenicity. It was proven that these vehicles induce only a slight increase of cytokine production in the spleen [110]. Additionally, the prime matter of MSNs, silica material, is generally recognized as a safe material with high biocompatibility, since it is being used in cosmetics and in food additive [111]. The MSNs have been proposed as delivery systems for low solubility drugs and for mediation of gene therapy. MSNs are internalized through endocytosis, but they are capable of endosomal escape and further delivery of their cargo inside the cytoplasm with maintenance of their morphology throughout the process; plus, no MSNs were detected in the nucleus [112].
AntagomiR-155 was delivered in colon cancer cells by integration in an MSN functionalized with polymerized dopamine (PDA) and AS1411 aptamer. Specifically, MSNs-NH2 were mixed with four OD anti-miR-155 at 4 °C, conjugated in order to obtain the complex: MSNs-anti-miR-155@PDA that was then covalently bound to AS1411 aptamer via the PDA film with the help of the -SH group. The uptake of anti-miR-155 via this nanoparticle complex was investigated in SW480 cells (at 6 h and at 12 h) by analyzing the level of FAM-labeled anti-miR-155, with increased uptake for the complex conjugated with the aptamer compared with the same complex but without the aptamer. Evaluation of cell viability showed that MSNs@PDA-Apt nanoparticles have almost no toxicity on cells. Differences in cell viability were observed in the case of MSNs-anti-miR-155@PDA with or without conjugated aptamers, with a higher degree of cell death in the case of MSNs-anti-miR-155@PDA-Apt. Also, the colony formation capacity was significantly decreased in the MSNs-anti-miR-155@PDA-Apt treated cells. At molecular level, there was a marked decrease in miR-155 and NF-κB (P65) at both mRNA and protein level. Intravenous injection of the complex in nude mice engrafted with SW480 cells showed increased fluorescence signal in the tumor, together with local malignant accumulation of the therapeutic complex. Tumor growth was significantly inhibited and no obvious changes were observed in the body weight or in the histological parameters of major organs. The same complex was tested in vitro and in vivo for reversal of drug resistance and there were improved results in terms of cell viability in the case of combined 5-FU and MSNs-anti-miR-155@PDA-Apt in SW480 cells compared to 5-FU alone [113]. The issue of drug resistance was approached also in resistant glioma cells via administration of a MSN that includes a Cy5 fluorophore in the silica, loaded with TMZ and conjugated on its surface with a polyarginine‐peptide nucleic acid (R8‐PNA) against miR-221. For comparison purposes, similar nanovehicles were concomitantly tested, containing only the TMZ drug (TMZ‐MSNPs) or only the R8‐PNA221 (PNA‐MSNPs) sequence. The TMZ resistant glioma cell line, T98G, treated with 0.5 mg mL−1 of PNA‐TMZ‐MSNPs showed an increased apoptotic rate—more precisely a percent of 70.9% apoptotic cells, a rate higher than the sum of the values obtained after treatment with MSNs conjugated only with PNA or the MSNs loaded only with TMZ. Also, a synergistic effect between the TMZ and anti‐miR221 PNA was observed [114]. A similar combination approach was tested in colorectal cancer (CRC) by combining the activity of miR-328 with the one of bevacizumab via a MSN that was also functionalized on the surface with an epithelial cell adhesion molecule aptamer (MSNs-miR328@PDA-PEG-Apt-Bev). This complex showed the highest reduction of cell viability and the highest ability to inhibit colony formation compared to the different combinations lacking part of the therapeutic complex. At molecular level, miR-328 level was found as increased, together with a reduction in CPTP, the target gene, in comparison with the control nanoparticle-treated cells. The in vivo distribution was evaluated in nude mice with CRC tumor xenografts (SW480 cell line), where the strongest intratumor fluorescence intensity was observed in the case of MSNs-IR-783@PDA-PEG-Apt-Bev compared with the free IR-783 dye, or with the dye incorporated in complexes without the Apt-Bev or without Bev. When applied as a treatment option, MSNs-miR-328@PDA-PEG-Apt-Bev presented the greatest capacity to induce growth inhibition of the xenografts with a high degree of necrosis observed in histological analysis. Finally, no major indications of systemic toxicity were observed, showing a good biocompatibility of the therapeutic system [115].
The main studies that used the MSNs as a delivery system for therapeutic miRNAs are included in Table 4.
Table 4.
The MSN-based miRNAs delivery and their therapeutic effects
| Cell lines | Loaded therapeutic agent | Biological effect | Molecular changes | Referenes |
|---|---|---|---|---|
| NCM460, SW480, HT-29, SW620, Lovo, Caco-2 | miR-328 | Decreased proliferation, tumorigenesis, small tumor size, no systemic toxicity | Downregulation of CPT | [115] |
| SW480, HT-29, SW620, Lovo, Caco-2, NCM460 | antimiR-155 | Decreased proliferation, tumorigenesis, small tumor size, no systemic toxicity | NfKB activation | [113] |
| MCF-7 | miR-21 | Controlled miRNA release | NA | [116] |
| HepG2/ADR | miR-375 + doxorubicine | Increased apoptosis, small tumor size, inhibited tumor cell proliferation, reduced size effects of doxorubicine | NA | [117] |
| C6 or T98G cells | temozolomide and antimiR-221 | Increased apoptosis | NA | [114] |
| HCT-116 and DLD-1 | paclitaxel and miR- 708 | Small tumor size | Downregulation of c-FLIP | [118] |
Quantum dots
Quantum dots (QDs) are very small NPs with dimensions ranging from 2 to 10 nm and highly versatile on their possible applications in medicine due to their unique optical and electronic properties; these nanoparticles can emit light with a specific frequency when stimulated with electricity or UV light. Depending on their size, material and shape, QDs specific emission has many application in cell imaging and diagnosis [119]. There are different types of QDs based on their composition (metal-containing or metal-free); the most common ones consist in cadmium (Cd)-containing semiconductor QDs, but there are also carbon QDs, germanium QDs, silicon QDs, silver chalcogenide QDs, black phosphorus QDs and polymer dots [119].
Semiconductor QDs are one of the most important categories in cancer imaging and therapy; their shape and size can be specifically controlled during the synthesis protocol and, therefore, the emission wavelength can be managed in concordance with their composition and size [120, 121]. Due to their rich surface chemistry, QDs can also function in cancer therapy as vehicles for drug delivery, generators of reactive oxygen species (ROS) or act as heat productors under irradiation toward disruption of cancer cells [119]. QDs showed high intratumor accumulation when bound to a specific tumor targeting molecule, such as a monoclonal antibodies (mAb) [122]. The application of QDs in cancer therapy was tested for the case of chemotherapeutic agents with enhanced effects in terms of cellular uptake and tumor targeting ability of the nanosystem. Examples of such applications consist in 3-mercapitalpropionicacid (MPA)-capped CdTe QDs (MPA-CdTe QDs) which were used in drug resistant leukemia cells for the delivery of daunorubicin (DNR) [123] or ZnO QDs coated by polymer shells prepared to capture Gd3+ ions and doxorubicin (DOX) which presented better therapeutic effect than the commercial DOX liposomes (DOXIL) [124].
As for miRNA-related applications, QDs have been increasingly studied for their role in imagining of the non-coding sequences, more precisely for evaluating the miRNA intracellular or intratumoral intake [125, 126]. QD-RNA-AuNP probe was mixed with chitosan (CS)/poly(γ-glutamic acid) (γ-PGA) complex for detection of the pre-miRNAs inside the cell; QD-RNA-AuNP probe was prepared through conjugation of thiolated RNA to AuNPs via Au–S bond, followed by binding of the 3′-end amine contained by the RNA to the carboxy group on the QD surface. Inside the cells and under the action of intracellular pH, the complex was liberated from the CS/γ-PGA complex and bound the pre-miRNA precursor target via the conjugated RNA. The hybridization product was further processed by intracellular RNase III Dicer that released the QDs from the Au nanoparticle, leading to fluorescence emission of QDs that can be sensed via confocal microscopy and extrapolated to quantify the level of pre-miRNA present inside the cell. The complex was successfully tested for the detection of pre-miRNA let-7a within breast cancer cell lines, MDA-MB231 and MCF-7 [127]. Graphene-P-gp loaded with miR-122-InP@ZnS quantum dots (GPMQNs) was used for intracellular delivery of miR-122 in liver tumor cells resistant to therapy. The synthetized nanocomposite reached a final dimension of approximately 300 nm and was applied in HepG2/ADM cells demonstrating improved efficiency in inducing cell death by increasing the level of miR-122 to a higher extend than in the case of standard miRNA transfection. Photothermal destruction was also obtained after applying a laser power density of 20 W cm−2 (no hyperthermia effect was observed in cells transfected with mir-122). The complex was also tested in vivo after the intravenous injection of 10 mg kg−1 of GPMQNs with increased accumulation in hepatic tumors and also demonstrated capacity to induce cell death − 68% apoptotic cells, compared to 34% apoptotic cells in the group treated with miR-122 alone. However, 23% of apoptotic cells were also present in the group treated with nonconjugated GPMQNs (and 8% in the control group that did not received any treatment). Besides therapeutic purposes, this nanocomplex provided a better imaging of the tumor in vivo since mice that were treated with the labeled GPMQNs could exhibit spontaneous fluorescence [128]. In another example, in HCC, a multifunctional nanoparticle formed of near-infrared (NIR) fluorescent quantum dots (QDs), hyaluronic acid (HA) and polyethyleneimine (PEI) conjugated via electrostatic interactions with anti-miR-27a led to the downregulation of oncogenic FOXO1 and PPAR-γ in the malignant cells both in vitro and in vivo. The cell proliferation rate was slowed down, and the apoptosis rate was stimulated. In vivo, this therapeutic strategy resulted in smaller tumor weight and volume, without any major organ damage [129].
The main studies that used the QDs as a delivery system for therapeutic miRNAs are included in Table 5.
Table 5.
The QD-based miRNAs delivery and their therapeutic effects
| Cell lines | Loaded therapeutic agent | Biological effect | Molecular changes | References |
|---|---|---|---|---|
| HepG2/ADM | miR-122 | Increased apoptosis, increased tumor and cellular uptake, smaller tumor size | NA | [128] |
| HepG2,HL-7702, NIH-3T3 | antimiR-27a and CD44 | Increased apoptosis, smaller tumor size, no systemic toxicity | Down-regulation of CYCLIN D1, Up-regulation of P21, BAX AND CASPASE-3, FOXO1, PPAR-Γ | [129] |
Metallic nanoparticles
Regarding metallic nanoparticles, miRNA/siRNA delivery can be achieved by attaching the molecules at the particle surface basing on their affinity for specific ligand bonds. Usually, the particles are biocompatibilized before functionalization in order to avoid these sequences’ rapid degradation before entering the cell.
Gold nanoparticles (AuNPs)
The use of gold NPs as carriers for various molecules including miRNA/siRNA is motivated by their unique physical–chemical properties (i.e. optical, electric), which distinguish them from the more often used polymeric or lipophilic counterparts. Gold nanoparticles present mechanical and chemical stability and an easily modifying surface which makes them amenable for a large variety of binding reactions [130–132]. In addition, gold NPs can be fabricated in a variety of shapes and aspect ratios [133–135]. Consequently, such distinct morphologies induce a variation in the total surface area of the particles, hence in their loading capacity [136, 137]. The synthesis of metallic nanoparticles is generally divided into two approaches: “top-down” and “bottom-up”; the process of “bottom-up” synthesis refers to the assembly from single atoms or molecules toward the construction of the nanoparticle, where the “top-down” one involves the disruption of a system or a molecule to obtain the nanoparticles [138]. Based on the above two methods, different chemical or physical approaches are currently employed to reach the synthesis of nanoparticles: microemulsion, microwave, thermal decomposition, sonochemical, polyol method, chemical reduction, laser ablation and others [131]. The decisive parameters that actually define in the end the role of the NPs in the targeted environment consists in size, shape and morphology that can be adapted based on the variations of various factors such as incubation time, temperature, surfactants used, concentration of the metal substrates, balance between metal precursor and reducing/stabilizing agent and others [131]. The morphological characteristics of gold nanoparticles were also shown to have a dramatic role on the physical properties, specifically on the optical properties of the particles. For instance, a variation of the morphology can shift the plasmonic response of the same material particle from the low-visible region (520 nm) for 20 nm gold nanospheres to the near-infrared (800 nm) in the case of high aspect ratio gold nanorods [108, 139], affording the simultaneous delivery of the agent and the in vivo imaging of the nanosystem at the targeted site. Analysis of the optical scattering properties of different sizes (20, 30 and 50 nm), shapes (spherical—GNSs and flower-shaped—GNFs) (Fig. 6) and surface conjugation (without and with PEG) gold nanoparticles showed that an expansion in the size of the nanostructures is correlated with an increase of the scattered intensity; moreover, GNFs were shown to have 1000-times higher scattered signal compared to GNSs of the same size [140]. All of the above-discussed properties might enable gold nanoparticles surpass other available delivery nanosystems.
Fig. 6.
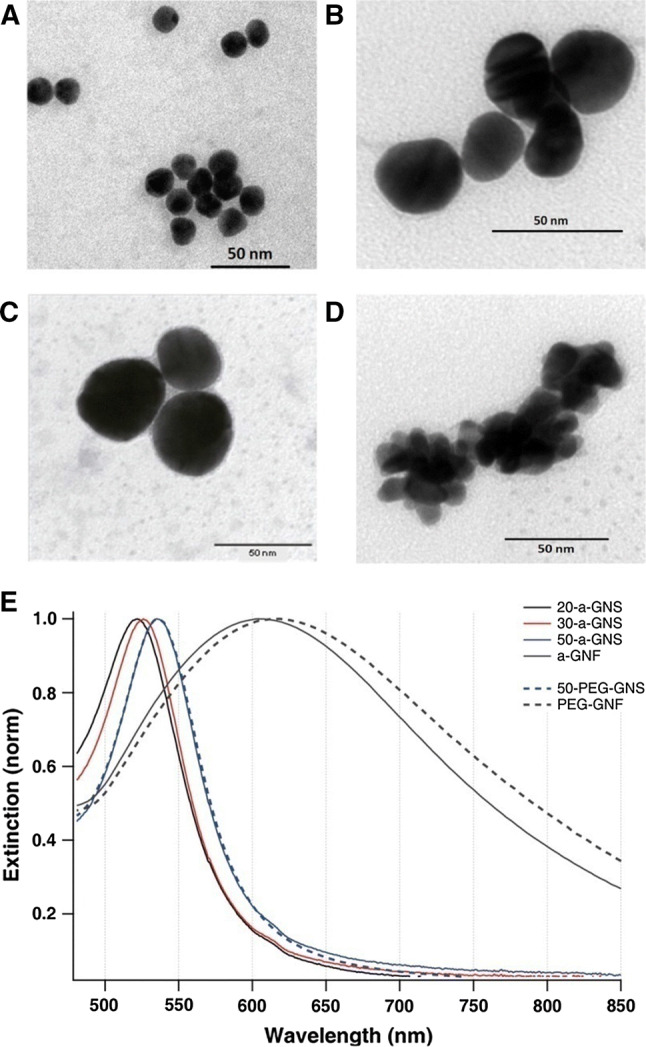
TEM visualization of a 20-a-GNS, b 30-a-GNS, c 50-a-GNS and d a-GNF and e extinction spectra for nanoparticles coated with PEG (dotted lines) and without PEG (solid lines).
Reprinted (adapted) with permission from Djaker et al. [140]
The first use of AuNPs as an in vitro gene delivery system based on electroporation in an osteoblast-like cell on microchips dates back to 2004 [141]. Five years later, gold nanoparticles were adopted as template for the design of miRNAs and anti-miRNAs nanodelivery systems for regulating the expression of multiple genes and controlling cellular behavior. Although from the class of noble metals, gold prevails as the choosing material for the design of nanocarriers of biomolecules, and this is mostly due to its general inherent low toxicity and nonimmunogenicity, potential cytotoxicity of the nanoparticles, including the Au ones is a matter of great interest before wide application in the clinic. Preliminary studies have shown encouraging results, but there is a fine line in designing and administrating the AuNPs to the point of minimal cytotoxicity and good therapeutic response. For example, the cytotoxicity of 5 nm and, respectively, 15 nm AuNPs, has been tested on mice fibroblast after 72 h of exposure and the results showed that NPs measuring 5 nm in a concentration of more than 50 μM were associated with cytotoxic effects, while 15 nm ones presented good biocompatibility. The smaller ones were associated with a role in disrupting the cytoskeletal organization of the fibroblasts [142]. The concentration of the AuNPs is also a decisive factor regarding the toxicity effects—AuNPs of 4 nm in doses higher than 200 nM impacted the cell viability through ROS generation, while administration of the same NPs in doses of 10 nM had no significant effects upon cell morphology, viability, ROS concentration and cytoskeleton organization [143]. A multi-parametric study on human endothelial cells (HUVEC) analyzed the cytotoxic effect of six different AuNP types: morphology—spherical (GNP) and flower-shaped (GNF), size - ~ 15 and ~ 50 nm diameter, surface chemistry—noncoated and PEGylated form. Cell viability was significantly influenced by doses of 10 pM for GNPs and 1 pM for GNFs ones; moreover, the most conclusive parameter for assessment of possible toxicity was represented by GNPs’ roughness and went independently on surface chemistry. Moreover, despite superior optical properties of the GNFs, their activity was associated with more deleterious effects [144].
In terms of strategies for miRNA modulation in cancer, 10 nm streptavidin-coated AuNPs were combined with QDs, a targeting MAb-ChL6 and an apoptosis-inducing miR-491 (MAb-GNP-miR491-Qdot). The study reported both a therapeutic effect and imaging capability of the nanocomposites [145]. Reinforcement of miR-205 (double-stranded alkylthiol-modified RNA molecules) immobilized on the surface of 13 ± 1 nm AuNPs was tested on PC-3 human prostate cancer cell line that has a low level of the reminded miRNA. The nanocomplex showed increased efficiency in downregulating the direct target of miR-205, PRKCε, by 52% in comparison with the control counterpart composed of non-targeting sequences bound to AuNPs. Moreover, the nanostructured induced apoptosis and inhibited migration in the treated cells. The same strategy was also tested for another miRNA with functional role in prostate cancer (oncogenic), miR-20a, with effects upon protection from apoptosis even under doxorubicin treatment [146]. In HCC, in vitro tests showed an efficient delivery of miR-375 by the AuNPs concomitant with effects such as suppressed invasion, colony formation and induced apoptosis. The complex was prepared through gold-sulfur covalent conjugation between miR-375 labeled with Cy3 at the 3′ end of the antisense strand and the gold nanoparticle followed by PEGylation for the stabilization of the complex. At the molecular level a decreased expression of BCL-2, ATG-7, YAP1 and an increased level of cleaved Caspase-3 were reported. As for in vivo experiments (xenograft tumors), the AuNP-miR-375 nanoparticles showed increase accumulation of the sequence in the tumor, impairment of carcinogenesis and no apparent toxicity to the mice [147].
As described in the previous example, AuNPs are usually preloaded with various surface molecules to facilitate miRNA binding. The pegylated AuNPs for miR-1 [148] and the cysteamine-functionalized AuNPs for miR-31 [149] were shown to deliver the therapeutic cargo more effectively than in the case of liposome-based delivery.
In a study outside the area of oncology, but with possible extrapolation to the cancer field, two miRNAs were conjugated onto plasmonic gold nanorods (AuNRs) which were previously functionalized with single-stranded DNA (ssDNA) for the linkage of miRNA via hybridization. The ssDNA chemistry and density were adapted in order to respond to different melting temperatures and therefore, to have different release profiles in the moment of stimulation with a NIR laser source. Cecropin mellitin (CM) was also used due to the demonstrated membrane-perturbing function and therefore, to enhance the uptake of the nanocomplex in the cells. The two miRNAs-AuNRs systems heated up to specific melting temperatures at: 51.7 °C for miR-155 complex and 68.9 °C in the case of the miR-302a-one. The phenomenon was also correlated with the laser power density: 1.25 Wcm−2 for miR-155 and 2 W cm−2 for miR-302a. For temporal release, HEK-293T reporter cells were incubated with both nanostructure types and irradiated for 2 min with different laser powers. Both miRNAs showed a laser power dependent release with the possibility of temporal control. The same strategy, once tested in the targeted cells, human outgrowth endothelial cells (OECs), was in line with the results from the reporter cells with functional changes related to the sustenance of angiogenesis. In vivo acute wound healing model sustained the beneficial effect found in vitro, but with important additional data referring to the order of miRNA release: miR302a-dsDNA51.7-AuNR and miR155-dsDNA68.9-AuNR (inverted ssDNA binding) presented the most efficient wound healing kinetics [150]. Similar sequential drug release systems were applied to malignant glioma. The loading of miR-218 mimic and temozolomide in anionic AuNPs (AuCOOH), which was then integrated into a FA-CS nanogels, effectively delivered the therapeutic cargo. The uptake of the negatively charged particle AuCOOH-miR218 mimics was increased by the FA-CS nanogel in both of the in vitro and the in vivo models. Temozolomide escaped the complex due to nanogel swelling through diffusion followed by the miR-218 release through exchange of GSH in cancer cells. This sequential delivery had a synergistic inhibitory effect upon U87MG glioblastoma cells and also good biocompatibility [151]. An alternative approach for miRNA delivery consisted in the transfection with a premiRNA, specifically premiR-145 expressing DNA plasmid conjugated with AuNP. The premiR-145 was successfully delivered into glioma cells, causing a decreased expression of CTGF [152]. AuNPs were also used for carrying EBV-encoded BART miRNAs for nasopharyngeal carcinoma (NPC) treatment in animal models, in combination with PEI/PEG polymers [153]. Furthermore, bioengineered delivery vectors are conjugated with targeting ligands in order to increase their intracellular uptake. Two such novel AuNPs systems were synthesized by Guo and his team [154]. Namely AuNPs-PEG-Tf (negatively charged AuNPs with the transferrin targeting ligands) were proved to successfully achieve receptor-mediated cellular uptake in PC-3 cells, a prostate cancer cell line highly expressing Tf receptors, while AuNPs-PEI-FA (positively charged AuNPs with the folate-receptor targeting ligands) effectively complexed small interfering RNA (siRNA) through electrostatic interaction. Following endolysosomal escape the AuNPs-PEI-FA.siRNA formulation produced enhanced endogenous gene silencing compared to the nontargeted formulation.
Iron oxide nanoparticles (IONPs)
Iron oxide nanoparticles (IONPs) are another viable option for the delivery of therapeutic miRNAs. These NPs have strong magnetic properties, high biocompatibility and are easily separated. However they can be easily oxidized, which is why they are usually coated with a silica or gold based material [155].
In pancreatic cancer, the IONPs were conjugated with an anti-CD44 Ab for a targeted delivery and with miRNA-21 antisense oligonucleotides. The intratumoral accumulation was greater and it resulted in smaller tumor size. In addition, impaired invasion and metastasis through increased levels of E-cadherin and decreased level of Vimentin was observed. It also stimulated the expression of PDCD4 and PTEN, while downregulating the anti-apoptotic gene BCL-2 [156]. In another study, the IONPs-mediated delivery of miR-145 combined with p127 resulted in inhibited proliferation, invasion, and tumorigenic capacity. The expression of the targeted genes: MUC13, pAKTSer473 and HER2 was inhibited and the expression level of TP53 was restored to homeostatic levels [157].
Core–shell nanoparticles
Core–shell NPs are a class of NPs that are generally composed of two or multiple materials/layers and are formed by an inner NPs core which is coated by an outer shell. Core–shell NPs come in a variety of shapes such as spherical, cubical, prismatic, hexagonal, wire or rod-shaped, etc. Each component of these complex nanoparticles has a unique set of physical and chemical properties that are combined in order to enhance the overall stability, bioavailability, proper clearance, higher specificity and higher control over drug release [158].
In regard to miRNAs applications, this type of NPs have been proposed as part of the miRNA detection strategies or for miRNAs delivery. One recent example of core–shell NPs conjugated to miRNAs involved the use of platinum hemispheres that were partially coated with Ag-thiol-miRNAs. When immobilised on an electrode surface in a nucleic acid sandwich assay, these particles generate significant electrocatalytic currents which boosts their sensitivity down to sub-femtomolar concentration of the targeted molecules. The system was proposed for the detection of miR-132 in the blood of neuroblastoma patients [159].
ZnFe2O4 magnetic nanoparticles coated by a mesoporous silica shell were proposed for the codelivery of let-7a and doxorubicin in chemoresistant breast cancer cells. The core–shell NPs were supplementary coated with polyethylenimine (PEI) which promotes the electrostatic interaction with the negatively charged miRNA. The high internalization of these NPs is facilitated by the aggressive phenotype of the cancer cells. Triple-negative breast cancer cells (MDA- MB-231) express a higher number of integrins on their surface as opposed to double positive breast cancer cells (MCF-7). Moreover, due to the fact that most of the outer surface of these core–shell NPs was coated with iPGD peptide, they can specifically target highly expressing integrin cells. Plus, the mesoporous silica shell is easily degraded under intracellular conditions, while by the external application of a magnetic field higher cell death is achieved due to the inner core heating [160].
Other complex metallic nanoparticles can be fabricated by coating the metallic core with various materials such as graphene. This type of nanoparticles was proved to have a greater controlled released of the therapeutic agent. In a study of breast cancer, gold nanorods (AuNRs) covered with graphene oxide were evaluated for the delivery of miR-101. The nanocomplex delivery application was combined with thermal therapy. The results showed that the up-regulation of miR-101 and decrease in cell viability was stronger in graphene oxide coated AuNRs than in other therapeutic systems [161].
Conjugation of metallic particles with another type of (nano)structures can be made in order to provide versatility and increase the therapeutic advantages. As for example, hollow gold nanoparticles (HGNPs) were linked to dendrimers and loaded with doxorubicin and antimiR-21. The whole nanocomposite lead to a controlled drug release, enhanced efficiency and the in vivo studies revealed a low systemic toxicity and high intratumor localization [162].
The main studies that used core–shell and complex metallic NPs as a delivery system for therapeutic miRNAs are included in Table 6.
Table 6.
The metallic NPs-based miRNA delivery and their therapeutic effects
| Cell lines | Loaded therapeutic agent | Biological effect | Molecular changes | References |
|---|---|---|---|---|
| Gold nanoparticles (AuNPs) | ||||
| Hep3B and HepG2 | miR-375 | Decreased invasion, colony formation, increased apoptosis, | Down-regulation of Bcl-2, ATG7, YAP1, Up-regulation of cleaved CASPASE 3 | [147] |
| Pca | miR-205 mimic | Decreased invasion, increased apoptosis (when associated with doxo) | Down-regulation of PTEN, E2F1 | [146] |
| U87MG, A549 | miR-208 mimic | Decreased cell viability, small tumor size | NA | [151] |
| Iron oxide nanoparticles (IONPs) | ||||
| C4-2, PC-3 | miR-205 | Increased chemosensitization, apoptosis, cell cycle arrest | Down-regulation of ZEB1, MED1, MMP2, MMP9, | [163] |
| PANC, Mia PaCa-2 | antimiR-21 | Increased invasion/metastasis, small tumor size | Up-regulation of E-CADHERIN, Up-regulation of VIMENTIN, PDCD4, PTEN, BAX, BCL-2 | [156] |
| HPAF-II, AsPC-1 | miR-145 | Decreased proliferation, clonogenicity, invasion, | Up-regulation of MUC13, pAKTSer473 and HER2, p53 | [157] |
| Complex metallic nanoparticles | ||||
| MCF7, MDA, HU02 | miR-101 | Decreased cell viability | NA | [161] |
| MDA-MB-231 and MCF-7 | let-7a, doxorubicin | Decreased chemoresistance, viability, increased apoptosis | NA | [160] |
| MDA-MB-231(A) and MCF-7 cells | anti-miR-21, doxorubicin | Increased apoptosis, high intratumor accumulation | NA | [162] |
DNA nanomaterials
Structural DNA nanotechnology has been ascending in the past 30 years by making use of the combination between stable branched DNA and sticky ended cohesion. Therefore, it is feasible to generate new materials based on DNA by combining the two above features in protocols of self-assembly [164]. Assembly of DNA is one of the most predictable and programmable protocol due to specificity of the binding and can be also generated with a wide variance of choices of sequences. Moreover, the thermodynamic stability is also an important feature. By means, simple branched molecules can form polyhedral constructs with edges represented by the double helical DNA and vertices by the branch points. Stiffer branched points can be exploited in the production of 2D and 3D periodic lattices of DNA (crystals). Whatever the construct, according to Nadrian C. Seeman [164], which also explicitly describes the context, the production of DNA nanomaterials is based on 3 main pillars: hybridization, stably branched DNA, and convenient synthesis of designed sequence. Paul Rothemund demonstrated in 2006 the principle of DNA origami by making use of a long, viral ‘scaffold’ single strand of DNA that can fold into a specific model through the addition of short ‘staple strands’ with roles in bringing together specific parts of the long ssDNA [165]. The application of DNA nanotechnology is continuously expanding and includes assembling inorganic nanostructures with DNA, assembling proteins with DNA, and biophysical and biomedical applications with emphasis on drug delivery and tissue engineering [166].
For drug delivery, the advantages of DNA nanostructures like wireframe DNA cages [167] and origami structures [168] consist in significant nuclease resistance and also stability. Even so, DNA origami structures can dissociate in environments with low magnesium, which affects their stability in vivo [169].
Intercalating antitumor drugs have been loaded on DNA origami structures; such examples include non-covalently attached doxorubicin on self-assembled DNA origami nanostructure via intercalation and delivery into breast adenocarcinoma cell line – MCF-7, with a high loading activity including doxorubicin resistant cancer cells. The accumulation in these last cells induced the reversal of the resistant phenotype. The authors speculated that the activity of doxorubicin-loaded DNA origami can impair the lysosomal acidification with effects upon redistribution of the active component to the action site [170]. Self-assembled DNA tetrahedral nanoparticles were used for the in vivo delivery of four siRNAs that ended with folate groups, demonstrating tumor targeting capacity and also gene silencing. The circulation in the blood compared the parent siRNA was approximately 4 times higher (t1/2 ≈ 24.2 min, compared to t1/2 ≈ 6 min). The authors also showed through the ease of programmable DNA complexes that the optimal delivery is achieved in the moment of at least three folate molecules addition and also in a specific spatial orientation of the ligands [171]. Further advancements are made by selectively designing the DNA nanostructures in order to target overexpressed molecules from the cancer environment (selective and specific targeting). In this sense, a prototype called “Logic-Gated Nanorobot” representing in fact a DNA origami cage connected to antibodies and closed by two aptamer switches was designed. After the encounter of a leukemic cell overexpressing the antigen protein, the DNA structure opened and released the cargo [172]. In line with the targeting specificity, Bujold et al. designed a DNA “nanosuitcase” that can incorporate oligonucleotide compounds like siRNA construct; furthermore, the release of the cargo is made upon recognition of an oligonucleotide trigger represented by amRNA or miRNA—synergistic therapy. Parts of the advantages, besides targeted delivery, consist in almost 100% yield, ability of cargo protection against nuclease degradation and also site-specific cleavage, and no toxicity [173].
Controlled release
Delivery vehicles from the nanotechnology niche helped in the overcoming of numerous clinical issues related to the short half-life of the therapeutic cargo or the diffuse and unspecific distribution, with potential toxicity upon healthy cells. Further investigations have underlined the potential of nanoparticles in controlled drug release for tunable and sustained delivery at the therapeutic spot [174]; the aspect of prolonged release over time is especially important in the case of miRNAs. Recently, Gulei et al. [175] highlighted the fact that miRNAs could better function as a first line of treatment by destabilization of the cancer cells followed by the standard therapy for the specific malignancy. The reason behind this assumption consists in the heterogeneous panel of miRNA target genes that can hold both tumor suppressor and oncogenic features (for the same miRNA) and modulation at such an extensive level can cause major side effects. In line with this, constant administration of minimal doses of miRNAs under controlled and time extended release could destabilize the malignant cell and make it more susceptible to the second line of treatment; also, installation of drug resistance could be limited. Moreover, miRNAs act upon the mRNAs that are continuously transcribed, especially in the case of overexpressed oncogenic genes. Therefore, their effect is only temporary if the cell survives at the first release of therapeutic miRNAs. Therefore, constant minimal doses of miRNAs released in the cell can overcome the consistently transcribed target transcripts until destabilization of the cancer cell occurs at such a degree that cannot overcome the second line of treatment. The same principle is applicable for the administration of miRNA inhibitors meant to eradicate the synthesized oncogenic non-coding transcript.
Regarding miRNAs and drug molecules release from these nanoparticulate systems, there are some strategies depending on the physical and chemical properties of the delivery system. The different synthesis protocols and routes of administration influence the in vitro or in vivo nanocarrier stability. Due to the fact that many protocols include an electrostatic interaction between the nanoparticle and the miRNA molecule, the stability of the system is ensured by the addition of polymers [176, 177] and the direct targeting property is achieved through cell penetrating peptides [178]. The controlled release of the therapeutic agents from polymers depends on the crystallinity, glass transition, solubility and molecular weight. Polymers have amorphous areas in which the drug is loaded, and crystalline areas, which offer stability. Glass transition refers to the fact that the crystallized areas of the polymer either have a rubber consistency or a glass consistency. The rubber state is more diffusible than the glass state. Also, the polymers have to contain a balance between hydrophilic and hydrophobic parts, which will allow their passage through various sites of the body. Moreover, polymers with high molecular weight are more hydrophobic and less affected by the first three described parameters. When reaching the site of delivery, the drug is released from the polymers with the help of diffusion, osmotic pressure or erosion of the polymer [179].
Many materials used for nanoparticle synthesis or coating surfaces dispose of stimuli responsiveness in order to target the desired cell compartment for a successful therapeutic effect. There are two main categories of stimuli: physical and chemical. Under the action of these stimuli, the nanoparticle structure is destabilized in order to break the chemical bonds and to release the therapeutic agent which was functionalized or encapsulated. Beside this role, stimuli have some influences in terms of therapy efficacy as a passive or an active one.
Physical stimuli induced controlled release profile
Physical stimuli include temperature, ultrasound, magnetic and electric field, and light. These stimuli are also called external stimuli [180].
Temperature responsive nanomaterials are the most studied and investigated ones regarding the controlled release of encapsulated molecules. Many nanoparticles and polymers dispose of structural properties which can be easily affected by temperature changes which decrease the toxicity and prolong their circulation time [181]. Tumor development implies inflammation processes accompanied by high temperature. Therefore, depending on the stimulus nature, temperature can be categorized as an internal or external stimulus. A lot of studies recommend the use of polymers for developing thermosensitive nanostructures for miRNA and siRNA delivery [182], especially hydrogels [183]. Louw et al. evaluated the in vitro therapeutic effect of miR-124 -chitosan polyplexes on microglia cells and in vivo effect on rat models for decreasing the inflammation of the spinal cord injury encountered in many neurodegenerative diseases [184]. Chitosan was proved to be very efficient as transfection agent for nucleic acids [185]. Considering this, many studies focused on chitosan hydrogels that are able to incorporate siRNA and drug molecules. These thermosensitive platforms dispose of liquid–solid phase transition and were used for in vivo breast cancer and melanoma studies by combining transglutaminase 2 siRNA and docotaxel therapeutic effect. The nanosystem proved superior results than siRNA alone, allowing temperature-dependent controlled release [186].
Ultrasound stimuli action involves an increased temperature at a specific tissue area in order to boost the permeability or to simply release the nanoparticle content [187]. For example, systemic administration of miR-127 bubble liposomes can determine the delivery of miR-127 molecules under in vivo ultrasound imaging on mouse models. Ultrasounds ensure the nanoparticles’ passive penetration inside the ischemic tissues. In addition, these liposomes can act as theranostic tools by performing diagnosis and therapy in ischemic diseases [41]. As for example, Wang et al. established an ultrasound-guided delivery of polymeric nanoparticles loaded with miR-122 into human colon cancer xenograft-bearing mice. This study proposes a promising targeted theranostic therapy [188].
Magnetic field application causes heat production which affects the nanosystem structure, increases tissue permeability and enhances the content release at the target site. The process of nucleic acid delivery assisted by magnetic field is well known as magnetofaction and is well studied as a therapeutic strategy [189–192]. The use of magnetic nanoparticles like colloidal iron oxide [193], metallic [194], bimetallic [195], ferrofluids [196], magnetosomes [197], magnetoliposomes [198]
and of late, smart hydrogels [199, 200] proved good results. However, the use of high-dose magnetic fields and the compatibility problems with the human body tissues are not encouraging their further use in clinical trials.
Electric field-responsive nanoparticles release their content under iontophoresis which activates the particles and modulates the cargo delivery. Such nanoparticles are usually anchored inside a smart hydrogel, creating a multi-stimuli responsive platform. For example, polymeric nanoparticles containing drug molecules are loaded into a thermo-sensitive hydrogel which is liquid at low temperature and gel at 37 °C body temperature. These mixtures were tested in vivo on animal models through subcutaneous injection. Electric field application ensured the release of the drug molecules from the nanoparticles and their diffusion from hydrogel pores in the surrounding tissue [201]. This particular example refers to a stimulation of a thermo-responsive carrier, but there are other two processes involved in the electric field-controlled release: oxidation reduction and structure disruption. Some designs focused on pulsatile release like smart nanoporous membranes [202]. These pulses can also affect the polymer networks leading to structure disruption and therapeutic agent delivery [203]. Despite the advantages presented above, electric field exposure may damage the healthy tissues and the effectiveness of this strategy may be diminished by other molecules which are present in the media composition [204, 205].
Light stimuli-responsive nanoparticles deliver their cargo after applying non-ionizing radiation and then proceeding with: photochemically triggered release, photoisomerization or photothermal release [206].
A photochemical reaction is induced after UV–Vis, NIR irradiation which cleaves the covalent bonds (e.g. ortho‐nitrobenzyl [207], coumarin [208], pyrede [209]) followed by the release of the cargo. NIR irradiation is preferred and strongly recommended because of superior deep tissue penetration [210]. Many studies have been conducted to find a suitable method to convert nanostructures such as liposomes [211] or micelles [212]) into light responsive ones. The solution found was by their functionalization with photosensitizers. When irradiated with light of suitable energy, these molecules generate reactive oxygen species which disrupt nanoparticle structure and release the therapeutic agent.
Photoisomerization reaction refers to reversible structural changes between isomers induced by UV–Vis light. In this case, the transition from trans to cis state describes a photo switchable process for controlled release [213]. The use of photoisomers represents the most appropriate choice in the framework of liposomes [214], micelles [215] and mesoporous silica nanoparticles [216]. A very disadvantageous fact is the limited number of photoisomerizable groups available for nanoparticle functionalization. Azobenzene groups are the most suggestive photoliable bonds [217, 218] that are used in developing new hybrid self-assembly structures for proteins and nucleic acid delivery [219]. These groups can be degraded by microenvironment enzymatic composition leading to toxic compounds’ formation (e.g. nitrobenzene) [220].
By photothermal reaction the therapeutic agent is released after light irradiation and concomitant heat production. The nanoparticle system has a dual role being composed of a photothermally active partner capable to convert the light energy into thermal energy and a thermoresponsive partner which disrupts the nanostructure and liberates the cargo.
Such an approach suggests the association of thermosensitive structures/molecules with plasmonic nanoparticles, therefore creating hybrid or multifunctional structures. For example, in such a complex the liposome can play the role of the thermoresponsive component while the gold nanoparticle has the role of the photoresponsive agent [221, 222].
In fact, gold nanoparticles were proved capable to respond to both of these stimuli. The NPs can be bound to double-stranded oligonucleotides, containing one strand of active miRNA and one strand of sensitizing miRNA. When near-infrared irradiation is applied, the sensitizing strand dehybridizes from the active strand thus releasing miRNA content inside the cell [223]. Zang P. et al. proposed the use of intrinsic pathways in order to amplify the tumor suppressor microRNA inside the cell. The nanosystem contained PEGylated gold-covered nanorods carrying DNA Y-motifs intercalated with doxorubicin. Inside the blood circulation, the protein corona protected the nanosystem from degradation. Due to the enhanced permeability and retention effect, the nanosystem accumulates at the tumor site, at which point a near-infrared (808 nm) laser beam is applied where the tumor is exposing the RGD proteins, which bind to the surface receptors found in tumor cells and allow the endocytosis of the nanoparticles. Once inside the cell, the siRNA and doxorubicin are released from the Y-motifs and induce mRNA silencing and inhibition of DNA synthesis, respectively. The endogenous microRNA uses what is left of the Y-motif to enter into a feedback-loop pathway, in which the microRNA is continuously amplified [224].
Chemical stimuli induced controlled release profile
Chemical stimuli are recognized as physiological stimuli and involve pH, redox potential, enzymes (e.g. matrix metalloproteinases) and some other different molecules that can interact with the nanoparticle and destabilize it leading to the release of the cargo [180].
The low pH level presented in the intratumoral environment represents an advantage for developing such nanodelivery systems based on polyelectrolytes, ester bounds and acetate bounds [225]. For example, in a mesoporous silica NP loaded with doxorubicin, miR-31 was added on the surface with the help of poly(ethyleneimine)/hyaluronic acid. The combined therapeutic effect was demonstrated in the case of HeLa cell line, human cervical cancer and H1299 cells, lung cancer. The in vivo studies indicated that the pH in the tumor microenvironment released the miRNA through the breakage of disulphide bounds [226].
Redox responsive nanoparticles’ action is based on the redox potential difference between healthy and unhealthy tissue and also between different cell compartments. The nanosystem design includes reduction or oxidation-susceptible bonds [227]. Deng et al. developed a self-assembly peptide-polysaccharide inter-polyelectrolyte structure conjugated with miR-34a and indocyanine green. Once the system enters the breast tumor cells, its disulfide bonds are disrupted because of intracellular redox potential of glutathione tripeptide and miR-34 is released [228]. Another example is represented by a co-delivery approach developed by Kang et al.. They coupled a hydrophobic drug like docetaxel with a hydrophilic shRNA for matrix metalloproteinase-9 into self-assembled polymeric micelles. These structures can be disassembled intracellularly in the presence of reducing agents, therefore liberating the drug and shRNA molecules. The study showed promising results in in vitro and in vivo breast cancer models [229].
Enzyme responsive nanoparticles aim to trigger specific chemical reactions in order to release their content [230]. The concentration of the enzyme is very important because it can decrease the drug toxicity [231]. On the other hand, the levels of several enzymes increase in pathological conditions like cancer. Matrix metalloproteinase-2 is involved in cancer progression and metastasis, representing a target for developing sensitive nanoparticles. Zhu et al. synthesized MMP-2 sensitive self-assembly polymeric micelles for co-delivery of siRNA and paclitaxel, which is a hydrophobic drug. These nanoparticles were tested in vitro and in vivo on lung cancer models of A549 cell line proving their passive targeting efficiency. The cellular uptake took place due to MMP-2 high-level presence, which cleaved the polymeric structure and then released siRNA and drug molecules. This method was proved to have also a transfection competence [232].
A very common problem encountered in transfection procedures is the negative charge of miRNA inhibitors, which risks their cellular uptake. Regarding improved molecule–nanosystem interaction that can favor the delivery of encapsulated compounds, many researchers recommend the use of hybrid systems. As for example, the conjugation of miR-204 inhibitors to gold nanoparticles dispersed in polymeric PLGA solution might represent a promising coating strategy for titanium implants used in regeneration. Herein, miRNAs’ efficient release is achieved due to polymer gradual disintegration, and their action increased the bone tissue cell differentiation [233]. Furthermore, many other studies that suggest the use of similar polymeric coating surfaces containing miRNA molecules (miR-29b and antimiR-138 [234], miR-21 [235]) for titanium implants’ easier acceptance and bone tissue regeneration are being currently in focus.
Concluding remarks
Choosing the proper miRNA nanodelivery design is a very challenging task because of the diversity of barriers encountered in the organism. The fact that miRNA molecules are small in size and low in weight represents an advantage in developing efficient transfection strategies. In this regard, a size under 100 nm and a neutral charge of the nanocarrier are experiencing a good tolerance in vivo [236]. Although the in vivo experiments repeatedly show low systemic toxicity and high accumulation inside the tumors, the long-term effects of this therapeutic approach are still poorly known.
Nowadays, there are a considerable number of preclinical studies which propose the use of nanodelivery systems for therapeutic miRNAs. The core center of the miRNA nanodelivery systems is still represented by liposomes, but their low specificity stimulates the exploration of alternative options. The exosomes are the closes option to liposomes, but because of their biological nature, they have a high risk of inducing an immune reaction. On the other hand, the polymer-based nanoparticles (dendrimers included) are a delivery system with great potential due to their versatility; however, the costs for their production still represent a major drawback. Many studies focused on using mesoporous silica nanoparticles due to their low toxicity, but their large size is a very inconvenient particularity. Even so, an alternative is to use this material as a coating surface for the metallic nanoparticles with an emphasis on noble metal nanoparticles (Au/Ag NPs) in order to increase their long-term biocompatibility. Moreover, the remarkable optical properties of these particles make them suitable for diagnosis purposes and many studies are exploring this background in order to develop nano-based theranostic agents.
An area of particular interest and another compelling miRNA delivery approach is represented by ultrasmall nanoparticles (metallic, quantum dots, carbon dots). Even if these nanostructures are generally applied as imaging tools due to their outstanding optical properties, they are also emerging as new system for the delivery of miRNAs.
Stimuli responsive materials dispose of unique physical and chemical properties and are highly recommended for performing a guided nanodelivery. In many cases the release of the cargo is influenced by a combination of stimuli leading to the development of multi-responsive or smart nanomaterials. Moreover, the release profile is controlled by the intensity and time of stimulus presence. It is very important to take into account the fact that the organism does not tolerate very well some stimuli exposure, especially the exogenous ones like temperature, light, magnetic or electric fields. In this regard, many nanoparticle syntheses are reoriented to acquire programmable features, therefore leading to the era of programmed nanomaterials [237]. These intelligent nanoconstructs may represent an excellent option for nucleic acid and drug molecule delivery. Anyhow, there are significant challenges in engineering and designing such programmable nanomaterials. Recent studies encourage the use of polymeric hydrogel platforms embedded with metallic nanoparticles conjugated with miRNAs. Moreover, the drug therapeutic effect can be enhanced when is co-delivered with miRNA [238]. These findings will provide insights into the complex scenario of personalized medicine.
Besides the advantages gained with polymeric fashioned nanoparticles, the futuristic designs will include nature-inspired nanoparticles that will be safer and will act more precisely based on biomimetic strategies. There are a few studies in this domain with promising results and green synthesis methods encourage the development of such new nanotools [239].
Acknowledgement
This work was supported by Competitivity Operational Program, 2014-2020, entitled “Clinical and economical impact of personalized targeted anti-microRNA therapies in reconverting lung cancer chemoresistance”—CANTEMIR, no. 35/01.09.2016, MySMIS 103375, Project PN-III-P1-1.2-PCCDI-2017-0782 entitled “Advanced innovative approaches for predictive regenerative medicine”- REGMED, no. 65PCCDI/2018 and project PNCDI III 2015-2020 entitled “Increasing the performance of scientific research and technology transfer in translational medicine through the formation of a new generation of young researchers” – ECHITAS, no. 29PFE/18.10.2018 and project CNFIS-FDI-2019-0666, entitled “Sustenance and valorification of research of excellence in the domain of personalized medicine by internationalization and increasement of research activities visibility”.
Compliance with ethical standards
Conflict of interest
Authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sanda Boca, Diana Gulei and Alina-Andreea Zimta contributed equally to this work.
Contributor Information
Rares Buiga, Email: raresbuiga@yahoo.fr.
Ioana Berindan-Neagoe, Email: ioana.neagoe@umfcluj.ro.
References
- 1.Dinger ME, Pang KC, Mercer TR, Mattick JS. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput Biol. 2008;4(11):e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starega-Roslan J, Krol J, Koscianska E, Kozlowski P, Szlachcic WJ, Sobczak K, et al. Structural basis of microRNA length variety. Nucleic Acids Res. 2011;39(1):257–268. doi: 10.1093/nar/gkq727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulei D, Irimie AI, Cojocneanu-Petric R, Schultze JL, Berindan-Neagoe I. Exosomes-small players big sound. Bioconjugate Chem. 2018;29(3):635–648. doi: 10.1021/acs.bioconjchem.8b00003. [DOI] [PubMed] [Google Scholar]
- 4.Braicu C, Catana C, Calin GA, Berindan-Neagoe I. NCRNA combined therapy as future treatment option for cancer. Curr Pharm Des. 2014;20(42):6565–6574. doi: 10.2174/1381612820666140826153529. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE. 2013;8(5):e62589. doi: 10.1371/journal.pone.0062589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Gulei D, Mehterov N, Nabavi SM, Atanasov AG, Berindan-Neagoe I. Targeting ncRNAs by plant secondary metabolites: the ncRNAs game in the balance towards malignancy inhibition. Biotechnol Adv. 2018;36(6):1779–1799. doi: 10.1016/j.biotechadv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redis RS, Berindan-Neagoe I, Pop VI, Calin GA. Non-coding RNAs as theranostics in human cancers. J Cell Biochem. 2012;113(5):1451–1459. doi: 10.1002/jcb.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braicu C, Calin GA, Berindan-Neagoe I. MicroRNAs and cancer therapy—from bystanders to major players. Curr Med Chem. 2013;20(29):3561–3573. doi: 10.2174/0929867311320290002. [DOI] [PubMed] [Google Scholar]
- 11.Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016;76(13):3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irimie AI, Braicu C, Sonea L, Zimta AA, Cojocneanu-Petric R, Tonchev K, et al. A looking-glass of non-coding RNAs in oral cancer. Int J Mol Sci. 2017;18(12):E2620. doi: 10.3390/ijms18122620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pop-Bica C, Pintea S, Cojocneanu-Petric R, Del Sal G, Piazza S, Wu ZH, et al. MiR-181 family-specific behavior in different cancers: a meta-analysis view. Cancer Metastasis Rev. 2018;37(1):17–32. doi: 10.1007/s10555-017-9714-9. [DOI] [PubMed] [Google Scholar]
- 14.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer—an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah MY, Calin GA. MicroRNAs as therapeutic targets in human cancers. Wiley Interdiscip Rev RNA. 2014;5(4):537–548. doi: 10.1002/wrna.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Roosbroeck K, Fanini F, Setoyama T, Ivan C, Rodriguez-Aguayo C, Fuentes-Mattei E, et al. Combining anti-Mir-155 with chemotherapy for the treatment of lung Cancers. Clin Cancer Res. 2017;23(11):2891–2904. doi: 10.1158/1078-0432.CCR-16-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Zhao H, Tan Z, Zhang C, Fu X. Bottleneck limitations for microRNA-based therapeutics from bench to the bedside. Pharmazie. 2015;70(3):147–154. [PubMed] [Google Scholar]
- 18.Baumann V, Winkler J. miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem. 2014;6(17):1967–1984. doi: 10.4155/fmc.14.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johannes L, Lucchino M. Current challenges in delivery and cytosolic translocation of therapeutic RNAs. Nucl Acid Ther. 2018;28(3):178–193. doi: 10.1089/nat.2017.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Haque F, Jasinski DL, Binzel DW, Shu D, Guo P. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018;414:57–70. doi: 10.1016/j.canlet.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Shushan D, Markovsky E, Gibori H, Tiram G, Scomparin A, Satchi-Fainaro R. Overcoming obstacles in microRNA delivery towards improved cancer therapy. Drug Deliv Transl Res. 2014;4(1):38–49. doi: 10.1007/s13346-013-0160-0. [DOI] [PubMed] [Google Scholar]
- 22.Jurj A, Braicu C, Pop LA, Tomuleasa C, Gherman CD, Berindan-Neagoe I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des Dev Therapy. 2017;11:2871–2890. doi: 10.2147/DDDT.S142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czauderna F, Fechtner M, Dames S, Aygun H, Klippel A, Pronk GJ, et al. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31(11):2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo BH, Bochkareva E, Bochkarev A, Mou TC, Gray DM. 2′-O-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro. Nucleic Acids Res. 2004;32(6):2008–2016. doi: 10.1093/nar/gkh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci U S A. 2000;97(10):5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyrup B, Nielsen PE. Peptide nucleic acids (PNA): synthesis, properties and potential applications. Bioorg Med Chem. 1996;4(1):5–23. doi: 10.1016/0968-0896(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 27.Pallan PS, Greene EM, Jicman PA, Pandey RK, Manoharan M, Rozners E, et al. Unexpected origins of the enhanced pairing affinity of 2′-fluoro-modified RNA. Nucleic Acids Res. 2011;39(8):3482–3495. doi: 10.1093/nar/gkq1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomuleasa C, Braicu C, Irimie A, Craciun L, Berindan-Neagoe I. Nanopharmacology in translational hematology and oncology. Int J Nanomed. 2014;9:3465–3479. doi: 10.2147/IJN.S60488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stylianopoulos T. EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors. Ther Del. 2013;4(4):421–423. doi: 10.4155/tde.13.8. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenesis Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iranian biomedical journal. 2016;20(1):1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–4244. doi: 10.1039/C6CS00636A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rappoport J, Preece J, Chipman K (2011) How do manufactured nanoparticles enter cells? UK Mathematics-in-Medicine Study Group Reading 2011, pp 1–33
- 37.Zhao J, Stenzel M. Entry of nanoparticles into cells: the importance of nanoparticle properties. Polym Chem. 2017;2018(9):259–272. [Google Scholar]
- 38.Urban-Morlan Z, Ganem-Rondero A, Melgoza-Contreras LM, Escobar-Chavez JJ, Nava-Arzaluz MG, Quintanar-Guerrero D. Preparation and characterization of solid lipid nanoparticles containing cyclosporine by the emulsification-diffusion method. Int J Nanomed. 2010;5:611–620. doi: 10.2147/IJN.S12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braicu C, Pileczki V, Irimie A, Berindan-Neagoe I. p53siRNA therapy reduces cell proliferation, migration and induces apoptosis in triple negative breast cancer cells. Mol Cell Biochem. 2013;381(1–2):61–68. doi: 10.1007/s11010-013-1688-5. [DOI] [PubMed] [Google Scholar]
- 41.Endo-Takahashi Y, Negishi Y, Nakamura A, Ukai S, Ooaku K, Oda Y, et al. Systemic delivery of miR-126 by miRNA-loaded bubble liposomes for the treatment of hindlimb ischemia. Sci Rep. 2014;4:3883. doi: 10.1038/srep03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HY, Mohammed KA, Kaye F, Sharma P, Moudgil BM, Clapp WL, et al. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. Int J Nanomed. 2013;8:4481–4494. doi: 10.2147/IJN.S41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu SY, Putral LN, Liang M, Chang HI, Davies NM, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharm Res. 2009;26(3):512–522. doi: 10.1007/s11095-008-9766-1. [DOI] [PubMed] [Google Scholar]
- 44.Maroof H, Islam F, Dong L, Ajjikuttira P, Gopalan V, McMillan NAJ, et al. Liposomal delivery of miR-34b-5p induced cancer cell death in thyroid carcinoma. Cells. 2018;7(12):265. doi: 10.3390/cells7120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piao L, Zhang M, Datta J, Xie X, Su T, Li H, et al. Lipid-based nanoparticle delivery of Pre-miR-107 inhibits the tumorigenicity of head and neck squamous cell carcinoma. Mol Therapy. 2012;20(6):1261–1269. doi: 10.1038/mt.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tolcher AW, Rodrigueza WV, Rasco DW, Patnaik A, Papadopoulos KP, Amaya A, et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(2):363–371. doi: 10.1007/s00280-013-2361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, et al. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J Controlled Release. 2014;173:43–50. doi: 10.1016/j.jconrel.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Peng F, Zhou T, Huang Y, Zhang L, Ye P, et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomed. 2015;10:4825–4836. doi: 10.2147/IJN.S79598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang T, Zhao P, Rong Z, Li B, Xue H, You J, et al. Anti-tumor efficiency of lipid-coated cisplatin nanoparticles co-loaded with MicroRNA-375. Theranostics. 2016;6(1):142–154. doi: 10.7150/thno.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu F, Liao JZ, Xiang GY, Zhao PX, Ye F, Zhao Q, et al. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017;6(3):651–661. doi: 10.1002/cam4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Y, Ornell KJ, Beliveau A, Jain A. Modulation of MicroRNAs 34a and 21 affects viability, senescence, and invasion in glioblastoma multiforme. J Biomed Nanotechnol. 2016;12(9):1782–1797. doi: 10.1166/jbn.2016.2274. [DOI] [PubMed] [Google Scholar]
- 53.Ando H, Asai T, Koide H, Okamoto A, Maeda N, Tomita K, et al. Advanced cancer therapy by integrative antitumor actions via systemic administration of miR-499. J Controlled Release. 2014;181:32–39. doi: 10.1016/j.jconrel.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Yung BC, Li J, Zhang M, Cheng X, Li H, Yung EM, et al. Lipid nanoparticles composed of quaternary amine-tertiary amine cationic lipid combination (QTsome) for therapeutic delivery of AntimiR-21 for lung cancer. Mol Pharm. 2016;13(2):653–662. doi: 10.1021/acs.molpharmaceut.5b00878. [DOI] [PubMed] [Google Scholar]
- 55.Xu S, Zhao N, Hui L, Song M, Miao ZW, Jiang XJ. MicroRNA-124-3p inhibits the growth and metastasis of nasopharyngeal carcinoma cells by targeting STAT3. Oncol Rep. 2016;35(3):1385–1394. doi: 10.3892/or.2015.4524. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Rajendran V, Kulshreshtha R, Ghosh PC. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int J Pharm. 2017;530(1–2):387–400. doi: 10.1016/j.ijpharm.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 57.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369(1652):20130502. doi: 10.1098/rstb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinf. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39(2):559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 62.Sempere LF, Keto J, Fabbri M (2017) Exosomal MicroRNAs in breast cancer towards diagnostic and therapeutic applications. Cancers (Basel). 9(7) [DOI] [PMC free article] [PubMed]
- 63.Pan JH, Zhou H, Zhao XX, Ding H, Li W, Qin L, et al. Role of exosomes and exosomal microRNAs in hepatocellular carcinoma: potential in diagnosis and antitumour treatments (Review) Int J Mol Med. 2018;41(4):1809–1816. doi: 10.3892/ijmm.2018.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stremersch S, Vandenbroucke RE, Van Wonterghem E, Hendrix A, De Smedt SC, Raemdonck K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Controlled Release. 2016;232:51–61. doi: 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20(3):380–390. doi: 10.1093/neuonc/nox152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Brien K, Lowry MC, Corcoran C, Martinez VG, Daly M, Rani S, et al. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6(32):32774–32789. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10(7):1517–1527. doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomed. 2018;13:585–599. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447–454. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreno-Vega A-I, Mez-Quintero T, Nuez-Anita R-E, et al. Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol. 2012;2012:10. doi: 10.1155/2012/936041. [DOI] [Google Scholar]
- 73.Mohamed A, Kunda NK, Ross K, Hutcheon GA, Saleem IY. Polymeric nanoparticles for the delivery of miRNA to treat chronic obstructive pulmonary disease (COPD) Eur J Pharm Biopharm. 2019;136:1–8. doi: 10.1016/j.ejpb.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun. 2016;7:10376. doi: 10.1038/ncomms10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathew AP, Cho KH, Uthaman S, Cho CS, Park IK. Stimuli-regulated smart polymeric systems for gene therapy. Polymers. 2017;9(4):E152. doi: 10.3390/polym9040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu XY, Wu DC, Li ZJ, Chen GQ. Polymer nanoparticles. Prog Mol Biol Transl Sci. 2011;104:299–323. doi: 10.1016/B978-0-12-416020-0.00007-3. [DOI] [PubMed] [Google Scholar]
- 77.Chiou GY, Cherng JY, Hsu HS, Wang ML, Tsai CM, Lu KH, et al. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J Controlled Release. 2012;159(2):240–250. doi: 10.1016/j.jconrel.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, Sadat SM. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccines Immunother. 2014;10(2):321–332. doi: 10.4161/hv.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Bertoni H, Kozielski KL, Rui Y, Lal B, Vaughan H, Wilson DR, et al. bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18(7):4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malhotra M, Sekar TV, Ananta JS, Devulapally R, Afjei R, Babikir HA, et al. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget. 2018;9(30):21478–21494. doi: 10.18632/oncotarget.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi: 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmerston Mendes L, Pan J, Torchilin VP. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):E1401. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: let’s meet the challenge. Int J Pharm. 2010;394(1–2):122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 85.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57(15):2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 86.Agrawal P, Gupta U, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28(22):3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252(1–2):263–266. doi: 10.1016/S0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 88.Luo D, Haverstick K, Belcheva N, Han E, Saltzman W (2002) Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA Deliv
- 89.Gajbhiye V, Vijayaraj Kumar P, Tekade RK, Jain NK. PEGylated PPI dendritic architectures for sustained delivery of H2 receptor antagonist. Eur J Med Chem. 2009;44(3):1155–1166. doi: 10.1016/j.ejmech.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Bhadra D, Yadav AK, Bhadra S, Jain NK. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int J Pharm. 2005;295(1–2):221–233. doi: 10.1016/j.ijpharm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 91.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257(1–2):111–124. doi: 10.1016/S0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 92.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magma. 2001;12(2–3):104–113. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 93.Agarwal A, Gupta U, Asthana A, Jain NK. Dextran conjugated dendritic nanoconstructs as potential vectors for anti-cancer agent. Biomaterials. 2009;30(21):3588–3596. doi: 10.1016/j.biomaterials.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 94.Conde J, Oliva N, Atilano M, Song HS, Artzi N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat Mater. 2016;15(3):353–363. doi: 10.1038/nmat4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu X, Li G, Su Z, Jiang Z, Chen L, Wang J, et al. Poly(amido amine) is an ideal carrier of miR-7 for enhancing gene silencing effects on the EGFR pathway in U251 glioma cells. Oncol Rep. 2013;29(4):1387–1394. doi: 10.3892/or.2013.2283. [DOI] [PubMed] [Google Scholar]
- 96.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tahiri-Alaoui A, Frigotto L, Manville N, Ibrahim J, Romby P, James W. High affinity nucleic acid aptamers for streptavidin incorporated into bi-specific capture ligands. Nucleic Acids Res. 2002;30(10):e45. doi: 10.1093/nar/30.10.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan W, Wang H, Chen Y, Zhang X, Zhu H, Yang C, et al. Molecular aptamers for drug delivery. Trends Biotechnol. 2011;29(12):634–640. doi: 10.1016/j.tibtech.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song KM, Lee S, Ban C. Aptamers and their biological applications. Sensors. 2012;12(1):612–631. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, Zhao X, Guo C, Ren D, Zhao Y, Xiao W, et al. Aptamer-dendrimer bioconjugates for targeted Delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS ONE. 2015;10(9):e0139136. doi: 10.1371/journal.pone.0139136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beavers KR, Werfel TA, Shen T, Kavanaugh TE, Kilchrist KV, Mares JW, et al. Porous silicon and polymer nanocomposites for delivery of peptide nucleic acids as anti-MicroRNA therapies. Adv Mater. 2016;28(36):7984–7992. doi: 10.1002/adma.201601646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maleki A, Kettiger H, Schoubben A, Rosenholm JM, Ambrogi V, Hamidi M. Mesoporous silica materials: from physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J Controlled Release. 2017;262:329–347. doi: 10.1016/j.jconrel.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Biol Med. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Mellaerts R, Aerts CA, Van Humbeeck J, Augustijns P, Van den Mooter G, Martens JA. Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials. Chem Commun. 2007;13:1375–1377. doi: 10.1039/b616746b. [DOI] [PubMed] [Google Scholar]
- 105.Heikkila T, Salonen J, Tuura J, Kumar N, Salmi T, Murzin DY, et al. Evaluation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Delivery. 2007;14(6):337–347. doi: 10.1080/10717540601098823. [DOI] [PubMed] [Google Scholar]
- 106.Kumar D, Sailaja Chirravuri SV, Shastri NR. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: a case study with aceclofenac. Int J Pharm. 2014;461(1–2):459–468. doi: 10.1016/j.ijpharm.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 107.Eren Z, Tunçer S, Gezer G, Yildirim L, Banerjee S, Yilmaz A. Improved solubility of celecoxib by inclusion in SBA-15 mesoporous silica: Drug loading in different solvents and release. Microporous Mesoporous Mater. 2016;235:211–223. doi: 10.1016/j.micromeso.2016.08.014. [DOI] [Google Scholar]
- 108.Jambhrunkar S, Qu Z, Popat A, Karmakar S, Xu C, Yu C. Modulating in vitro release and solubility of griseofulvin using functionalized mesoporous silica nanoparticles. J Colloid Interface Sci. 2014;434:218–225. doi: 10.1016/j.jcis.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 109.Guo Z, Liu XM, Ma L, Li J, Zhang H, Gao YP, et al. Effects of particle morphology, pore size and surface coating of mesoporous silica on Naproxen dissolution rate enhancement. Colloids Surf B. 2013;101:228–235. doi: 10.1016/j.colsurfb.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 110.Heidegger S, Gossl D, Schmidt A, Niedermayer S, Argyo C, Endres S, et al. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8(2):938–948. doi: 10.1039/C5NR06122A. [DOI] [PubMed] [Google Scholar]
- 111.Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7(7):E189. doi: 10.3390/nano7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta pharmaceutica Sinica B. 2018;8(2):165–177. doi: 10.1016/j.apsb.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y, Duo Y, Bi J, Zeng X, Mei L, Bao S, et al. Targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int J Nanomed. 2018;13:1241–1256. doi: 10.2147/IJN.S158290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bertucci A, Prasetyanto EA, Septiadi D, Manicardi A, Brognara E, Gambari R, et al. Combined delivery of temozolomide and anti-miR221 PNA using mesoporous silica nanoparticles induces apoptosis in resistant glioma cells. Small. 2015;11(42):5687–5695. doi: 10.1002/smll.201500540. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Duo Y, Zhai P, He L, Zhong K, Zhang Y et al (2018) Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine (Lond Engl) [DOI] [PubMed]
- 116.Deng K, Zhang Y, Tong X. Sensitive electrochemical detection of microRNA-21 based on propylamine-functionalized mesoporous silica with glucometer readout. Anal Bioanal Chem. 2018;410(7):1863–1871. doi: 10.1007/s00216-018-0859-3. [DOI] [PubMed] [Google Scholar]
- 117.Xue H, Yu Z, Liu Y, Yuan W, Yang T, You J, et al. Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma. Int J Nanomed. 2017;12:5271–5287. doi: 10.2147/IJN.S135306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gupta B, Ruttala HB, Poudel BK, Pathak S, Regmi S, Gautam M, et al. Polyamino acid layer-by-layer (LbL) constructed silica-supported mesoporous titania nanocarriers for stimuli-responsive delivery of microRNA 708 and paclitaxel for combined chemotherapy. ACS Appl Mater Interfaces. 2018;10(29):24392–24405. doi: 10.1021/acsami.8b06642. [DOI] [PubMed] [Google Scholar]
- 119.Wu F-G, Zhang X, Chen X, Sun W, Bao Y-W, Hua X-W, et al. Quantum dots for cancer therapy and bioimaging. In: Gonçalves G, Tobias G, et al., editors. Nanooncology, Nanomedicine and Nanotoxicology. Cham: Springer; 2018. [Google Scholar]
- 120.Vu TQ, Lam WY, Hatch EW, Lidke DS. Quantum dots for quantitative imaging: from single molecules to tissue. Cell Tissue Res. 2015;360(1):71–86. doi: 10.1007/s00441-014-2087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou J, Yang Y, Zhang CY. Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem Rev. 2015;115(21):11669–11717. doi: 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- 122.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 123.Zhou Y, Shi L, Li Q, Jiang H, Lv G, Zhao J, et al. Imaging and inhibition of multi-drug resistance in cancer cells via specific association with negatively charged CdTe quantum dots. Biomaterials. 2010;31(18):4958–4963. doi: 10.1016/j.biomaterials.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 124.Ye DX, Ma YY, Zhao W, Cao HM, Kong JL, Xiong HM, et al. ZnO-based nanoplatforms for labeling and treatment of mouse tumors without detectable toxic side effects. ACS Nano. 2016;10(4):4294–4300. doi: 10.1021/acsnano.5b07846. [DOI] [PubMed] [Google Scholar]
- 125.Daneshpour M, Karimi B, Omidfar K. Simultaneous detection of gastric cancer-involved miR-106a and let-7a through a dual-signal-marked electrochemical nanobiosensor. Biosens Bioelectron. 2018;109:197–205. doi: 10.1016/j.bios.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 126.Qu X, Jin H, Liu Y, Sun Q. Strand displacement amplification reaction on quantum dot-encoded silica bead for visual detection of multiplex MicroRNAs. Anal Chem. 2018;90(5):3482–3489. doi: 10.1021/acs.analchem.7b05235. [DOI] [PubMed] [Google Scholar]
- 127.Geng Y, Lin D, Shao L, Yan F, Ju H. Cellular delivery of quantum dot-bound hybridization probe for detection of intracellular pre-MicroRNA using chitosan/Poly(γ-Glutamic Acid) complex as a carrier. PLoS ONE. 2013;8(6):e65540. doi: 10.1371/journal.pone.0065540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeng X, Yuan Y, Wang T, Wang H, Hu X, Fu Z, et al. Targeted imaging and induction of apoptosis of drug-resistant hepatoma cells by miR-122-loaded graphene-InP nanocompounds. J Nanobiotechnol. 2017;15(1):9. doi: 10.1186/s12951-016-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng X, Zhang F, Zhao Y, Zhang J, Dawulieti J, Pan Y, et al. Self-assembled dual fluorescence nanoparticles for CD44-targeted delivery of anti-miR-27a in liver cancer theranostics. Theranostics. 2018;8(14):3808–3823. doi: 10.7150/thno.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stanley Rosarin F, Mirunalini S. Nobel metallic nanoparticles with novel biomedical properties. J Bioanal Biomed. 2011;3:085–091. [Google Scholar]
- 131.Singla R, Guliani A, Kumari A. Yadav S. Met Nanoparticles: Toxicity issues and applications in medicine; 2016. pp. 41–80. [Google Scholar]
- 132.Petrushev B, Boca S, Simon T, Berce C, Frinc I, Dima D, et al. Gold nanoparticles enhance the effect of tyrosine kinase inhibitors in acute myeloid leukemia therapy. Int J Nanomed. 2016;11:641–660. doi: 10.2147/IJN.S94064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nagy-Simon T, Tatar AS, Craciun AM, Vulpoi A, Jurj MA, Florea A, et al. Antibody conjugated, raman tagged hollow gold-silver nanospheres for specific targeting and multimodal dark-field/SERS/two photon-FLIM imaging of CD19(+) B lymphoblasts. ACS Appl Mater Interfaces. 2017;9(25):21155–21168. doi: 10.1021/acsami.7b05145. [DOI] [PubMed] [Google Scholar]
- 134.Boca-Farcau S, Potara M, Simon T, Juhem A, Baldeck P, Astilean S. Folic acid-conjugated, SERS-labeled silver nanotriangles for multimodal detection and targeted photothermal treatment on human ovarian cancer cells. Mol Pharm. 2014;11(2):391–399. doi: 10.1021/mp400300m. [DOI] [PubMed] [Google Scholar]
- 135.Boca S, Rugina D, Pintea A, Barbu-Tudoran L, Astilean S. Flower-shaped gold nanoparticles: synthesis, characterization and their application as SERS-active tags inside living cells. Nanotechnology. 2011;22(5):055702. doi: 10.1088/0957-4484/22/5/055702. [DOI] [PubMed] [Google Scholar]
- 136.Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials. 2011;1(1):31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satomi T, Nagasaki Y, Kobayashi H, Otsuka H, Kataoka K. Density control of poly(ethylene glycol) layer to regulate cellular attachment. Langmuir. 2007;23(12):6698–6703. doi: 10.1021/la0624384. [DOI] [PubMed] [Google Scholar]
- 138.Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 139.Boca SC, Astilean S. Detoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as SERS-active carriers of Raman tags. Nanotechnology. 2010;21(23):235601. doi: 10.1088/0957-4484/21/23/235601. [DOI] [PubMed] [Google Scholar]
- 140.Djaker N, Sultana S, Issaad D, Boca S, Moustaoui H, Spadavecchia J, et al. Spherical and flower-shaped gold nanoparticles characterization by scattering correlation spectroscopy. J Phys Chem C. 2016;120(21):11700–11708. doi: 10.1021/acs.jpcc.6b02436. [DOI] [Google Scholar]
- 141.Jen C-P, Chen Y-H, Fan C-S, Yeh C-S, Lin Y-C, Shieh D-B, et al. A Nonviral transfection approach in vitro: the design of a gold nanoparticle vector joint with microelectromechanical systems. Langmuir. 2004;20(4):1369–1374. doi: 10.1021/la036154k. [DOI] [PubMed] [Google Scholar]
- 142.Coradeghini R, Gioria S, Garcia CP, Nativo P, Franchini F, Gilliland D, et al. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett. 2013;217(3):205–216. doi: 10.1016/j.toxlet.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 143.Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, et al. Cytotoxic effects of gold nanoparticles: a multiparametric study. ACS Nano. 2012;6(7):5767–5783. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 144.Sultana S, Djaker N, Boca-Farcau S, Salerno M, Charnaux N, Astilean S, et al. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology. 2015;26(5):055101. doi: 10.1088/0957-4484/26/5/055101. [DOI] [PubMed] [Google Scholar]
- 145.Natarajan A, Venugopal SK, DeNardo SJ, Zern MA, editors. Breast cancer targeting novel microRNA-nanoparticles for imaging. SPIE BiOS; 2009: SPIE
- 146.Hao L, Patel PC, Alhasan AH, Giljohann DA, Mirkin CA. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small. 2011;7(22):3158–3162. doi: 10.1002/smll.201101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xue HY, Liu Y, Liao JZ, Lin JS, Li B, Yuan WG, et al. Gold nanoparticles delivered miR-375 for treatment of hepatocellular carcinoma. Oncotarget. 2016;7(52):86675–86686. doi: 10.18632/oncotarget.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghosh R, Singh LC, Shohet JM, Gunaratne PH. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials. 2013;34(3):807–816. doi: 10.1016/j.biomaterials.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 149.Ghosh R, Singh LC, Shohet JM, Gunaratne PH. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials. 2013;34(3):807–816. doi: 10.1016/j.biomaterials.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 150.Lino MM, Simoes S, Vilaca A, Antunes H, Zonari A, Ferreira L (2018) Modulation of angiogenic activity by light-activatable miRNA-loaded nanocarriers. ACS Nano [DOI] [PubMed]
- 151.Fan L, Yang Q, Tan J, Qiao Y, Wang Q, He J, et al. Dual loading miR-218 mimics and Temozolomide using AuCOOH@FA-CS drug delivery system: promising targeted anti-tumor drug delivery system with sequential release functions. J Exp Clin Cancer Res. 2015;34:106. doi: 10.1186/s13046-015-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Benisvy-Aharonovich E, Shimanovich U, Kronfeld N, Giladi N, Bier A, Kazimirsky G, et al. Pre-miRNA expressing plasmid delivery for anti-cancer therapy. MedChemComm. 2014;5(4):459–462. doi: 10.1039/C3MD00314K. [DOI] [Google Scholar]
- 153.Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X, et al. Gold nano-particles (AuNPs) carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive nasopharyngeal carcinoma. Oncotarget. 2015;6(10):7838–7850. doi: 10.18632/oncotarget.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo J, O’Driscoll CM, Holmes JD, Rahme K. Bioconjugated gold nanoparticles enhance cellular uptake: a proof of concept study for siRNA delivery in prostate cancer cells. Int J Pharm. 2016;509(1–2):16–27. doi: 10.1016/j.ijpharm.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 155.Ali A, Zafar H, Zia M, Ul Haq I, Phull AR, Ali JS, et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016;9:49–67. doi: 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y, Chen Y, Li J, Zhang Z, Huang C, Lian G, et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017;108(7):1493–1503. doi: 10.1111/cas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Setua S, Khan S, Yallapu MM, Behrman SW, Sikander M, Khan SS, et al. Restitution of Tumor Suppressor MicroRNA-145 Using Magnetic Nanoformulation for Pancreatic Cancer Therapy. J Gastrointest Surg. 2017;21(1):94–105. doi: 10.1007/s11605-016-3222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ghosh Chaudhuri R, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012;112(4):2373–2433. doi: 10.1021/cr100449n. [DOI] [PubMed] [Google Scholar]
- 159.Spain E, Adamson K, Elshahawy M, Bray I, Keyes TE, Stallings RL, et al. Hemispherical platinum: silver core: shell nanoparticles for miRNA detection. Anal. 2017;142(5):752–762. doi: 10.1039/C6AN02609E. [DOI] [PubMed] [Google Scholar]
- 160.Yin PT, Pongkulapa T, Cho HY, Han J, Pasquale NJ, Rabie H, et al. Overcoming chemoresistance in cancer via combined MicroRNA therapeutics with anticancer drugs using multifunctional magnetic core-shell nanoparticles. ACS Appl Mater Interfaces. 2018;10(32):26954–26963. doi: 10.1021/acsami.8b09086. [DOI] [PubMed] [Google Scholar]
- 161.Assali A, Akhavan O, Adeli M, Razzazan S, Dinarvand R, Zanganeh S, et al. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomedicine. 2018;14(6):1891–1903. doi: 10.1016/j.nano.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 162.Ren Y, Wang R, Gao L, Li K, Zhou X, Guo H, et al. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. Journal of Controlled Release. 2016;228:74–86. doi: 10.1016/j.jconrel.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 163.Nagesh PKB, Chowdhury P, Hatami E, Boya VKN, Kashyap VK, Khan S et al (2018) miRNA-205 nanoformulation sensitizes prostate cancer cells to chemotherapy. Cancers (Basel) 10(9) [DOI] [PMC free article] [PubMed]
- 164.Seeman NC. Nanomaterials based on DNA. Annu Rev Biochem. 2010;79:65–87. doi: 10.1146/annurev-biochem-060308-102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 166.Seeman NC, Sleiman HF. DNA nanotechnology. Nat Rev Mater. 2017;3:17068. doi: 10.1038/natrevmats.2017.68. [DOI] [Google Scholar]
- 167.Conway JW, McLaughlin CK, Castor KJ, Sleiman H. DNA nanostructure serum stability: greater than the sum of its parts. Chem Commun. 2013;49(12):1172–1174. doi: 10.1039/c2cc37556g. [DOI] [PubMed] [Google Scholar]
- 168.Mei Q, Wei X, Su F, Liu Y, Youngbull C, Johnson R, et al. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 2011;11(4):1477–1482. doi: 10.1021/nl1040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hahn J, Wickham SF, Shih WM, Perrault SD. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano. 2014;8(9):8765–8775. doi: 10.1021/nn503513p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, et al. DNA origami as a carrier for circumvention of drug resistance. J Am Chem Soc. 2012;134(32):13396–13403. doi: 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- 171.Lee H, Lytton-Jean AKR, Chen Y, Love KT, Park AI, Karagiannis ED, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335(6070):831–834. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 173.Bujold KE, Hsu JCC, Sleiman HF. Optimized DNA “nanosuitcases” for encapsulation and conditional release of siRNA. J Am Chem Soc. 2016;138(42):14030–14038. doi: 10.1021/jacs.6b08369. [DOI] [PubMed] [Google Scholar]
- 174.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gulei D, Berindan-Neagoe I. Combined Therapy in Cancer: the Non-coding Approach. Molecular therapy Nucleic acids. 2018;12:787–792. doi: 10.1016/j.omtn.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wen MM. Getting miRNA therapeutics into the target cells for neurodegenerative diseases: a mini-review. Front Mol Neurosci. 2016;9:129. doi: 10.3389/fnmol.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chaudhary V, Jangra S, Yadav NR. Nanotechnology based approaches for detection and delivery of microRNA in healthcare and crop protection. J Nanobiotechnol. 2018;16(1):40. doi: 10.1186/s12951-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci USA. 2012;109(26):E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y, Sun W, Gu Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J Controlled Release. 2014;194:1–19. doi: 10.1016/j.jconrel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, et al. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41(21):7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 182.Li Y, Gao J, Zhang C, Cao Z, Cheng D, Liu J, et al. Stimuli-responsive polymeric nanocarriers for efficient gene delivery. Top Curr Chem. 2017;375(2):27. doi: 10.1007/s41061-017-0119-6. [DOI] [PubMed] [Google Scholar]
- 183.Wang LL, Burdick JA (2017) Engineered hydrogels for local and sustained delivery of RNA-interference therapies. Adv Healthc Mater 6(1) [DOI] [PMC free article] [PubMed]
- 184.Louw AM, Kolar MK, Novikova LN, Kingham PJ, Wiberg M, Kjems J, et al. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomed Nanotechnol Biol Med. 2016;12(3):643–653. doi: 10.1016/j.nano.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 185.Zhao QQ, Chen JL, Lv TF, He CX, Tang GP, Liang WQ, et al. N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/DNA complex. Biol Pharm Bull. 2009;32(4):706–710. doi: 10.1248/bpb.32.706. [DOI] [PubMed] [Google Scholar]
- 186.Han HD, Mora EM, Roh JW, Nishimura M, Lee SJ, Stone RL, et al. Chitosan hydrogel for localized gene silencing. Cancer Biol Ther. 2011;11(9):839–845. doi: 10.4161/cbt.11.9.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gourevich D, Gerold B, Arditti F, Xu D, Liu D, Volovick A, et al. Ultrasound activated nano-encapsulated targeted drug delivery and tumour cell poration. Adv Exp Med Biol. 2012;733:135–144. doi: 10.1007/978-94-007-2555-3_13. [DOI] [PubMed] [Google Scholar]
- 188.Wang TY, Choe JW, Pu K, Devulapally R, Bachawal S, Machtaler S, et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. Journal of Controlled Release. 2015;203:99–108. doi: 10.1016/j.jconrel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9(2):102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 190.Prosen L, Prijic S, Music B, Lavrencak J, Cemazar M, Sersa G. Magnetofection: a reproducible method for gene delivery to melanoma cells. Biomed Res Int. 2013;2013:209452. doi: 10.1155/2013/209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Laurentt N, Sapet C, Le Gourrierec L, Bertosio E, Zelphati O. Nucleic acid delivery using magnetic nanoparticles: the Magnetofection technology. Therapeutic delivery. 2011;2(4):471–482. doi: 10.4155/tde.11.12. [DOI] [PubMed] [Google Scholar]
- 192.Oral O, Cikim T, Zuvin M, Unal O, Yagci-Acar H, Gozuacik D, et al. Effect of varying magnetic fields on targeted gene delivery of nucleic acid-based molecules. Ann Biomed Eng. 2015;43(11):2816–2826. doi: 10.1007/s10439-015-1331-6. [DOI] [PubMed] [Google Scholar]
- 193.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013;13(3):1059–1064. doi: 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Srinoi P, Chen Y-T, Vittur V, Marquez M. Randall Lee T. Bimet Nanoparticles: Enhanced magnetic and optical properties for emerging biological applications; 2018. p. 1106. [Google Scholar]
- 196.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, et al. Monodisperse MFe2O4 (M=Fe Co, Mn) nanoparticles. J Am Chem Soc. 2004;126(1):273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 197.Sun J, Li Y, Liang XJ, Wang PC. Bacterial magnetosome: a novel biogenetic magnetic targeted drug carrier with potential multifunctions. J Nanomater. 2011;2011(2011):469031–469043. doi: 10.1155/2011/469031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nappini S, Fogli S, Castroflorio B, Bonini M, Bombelli F, Baglioni P. Magnetic field responsive drug release from magnetoliposomes in biological fluids. J Mater Chem B. 2015;4:716. doi: 10.1039/C5TB02191J. [DOI] [PubMed] [Google Scholar]
- 199.Li J, Mo L, Lu CH, Fu T, Yang HH, Tan W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem Soc Rev. 2016;45(5):1410–1431. doi: 10.1039/C5CS00586H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jalili NA, Muscarello M, Gaharwar AK. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng Transl Med. 2016;1(3):297–305. doi: 10.1002/btm2.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ge J, Neofytou E, Cahill TJ, 3rd, Beygui RE, Zare RN. Drug release from electric-field-responsive nanoparticles. ACS Nano. 2012;6(1):227–233. doi: 10.1021/nn203430m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jeon G, Yang SY, Byun J, Kim JK. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011;11(3):1284–1288. doi: 10.1021/nl104329y. [DOI] [PubMed] [Google Scholar]
- 203.Servant A, Bussy C, Al-Jamal K, Kostarelos K. Design, engineering and structural integrity of electro-responsive carbon nanotube- based hydrogels for pulsatile drug release. J Mater Chem B. 2013;1:4593–4600. doi: 10.1039/c3tb20614a. [DOI] [PubMed] [Google Scholar]
- 204.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 205.Brudno Y, Mooney DJ. On-demand drug delivery from local depots. Journal of Controlled Release. 2015;219:8–17. doi: 10.1016/j.jconrel.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 206.Linsley CS, Wu BM. Recent advances in light-responsive on-demand drug-delivery systems. Ther Deliv. 2017;8(2):89–107. doi: 10.4155/tde-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao H, Sterner ES, Bryan Coughlin E, Theato P. o-Nitrobenzyl alcohol derivatives: opportunities in polymer and materials science. Macromolecules. 2012;45(4):1723–1736. doi: 10.1021/ma201924h. [DOI] [Google Scholar]
- 208.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1(7):441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 209.Wang H, Zhang W, Gao C. Shape transformation of light-responsive pyrene-containing micelles and their influence on cytoviability. Biomacromol. 2015;16(8):2276–2281. doi: 10.1021/acs.biomac.5b00497. [DOI] [PubMed] [Google Scholar]
- 210.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 211.Puri A. Phototriggerable liposomes: current research and future perspectives. Pharmaceutics. 2013;6(1):1–25. doi: 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang Y, Dong R, Zhu X, Yan D. Photo-responsive polymeric micelles. Soft Matter. 2014;10(33):6121–6138. doi: 10.1039/C4SM00871E. [DOI] [PubMed] [Google Scholar]
- 213.Bleger D, Hecht S. Visible-light-activated molecular switches. Angew Chem. 2015;54(39):11338–11349. doi: 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- 214.Cui ZK, Phoeung T, Rousseau PA, Rydzek G, Zhang Q, Bazuin CG, et al. Nonphospholipid fluid liposomes with switchable photocontrolled release. Langmuir ACS J Surf Colloids. 2014;30(36):10818–10825. doi: 10.1021/la502131h. [DOI] [PubMed] [Google Scholar]
- 215.Shen H, Zhou M, Zhang Q, Keller A, Shen Y. Zwitterionic light-responsive polymeric micelles for controlled drug delivery. Colloid Polym Sci. 2015;293:1685. doi: 10.1007/s00396-015-3550-7. [DOI] [Google Scholar]
- 216.Liu J, Bu W, Pan L, Shi J. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew Chem. 2013;52(16):4375–4379. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 217.Dong M, Babalhavaeji A, Samanta S, Beharry AA, Woolley GA. Red-shifting azobenzene photoswitches for in vivo use. Acc Chem Res. 2015;48(10):2662–2670. doi: 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- 218.Yao C, Wang P, Li X, Hu X, Hou J, Wang L, et al. Near-infrared-triggered azobenzene-liposome/upconversion nanoparticle hybrid vesicles for remotely controlled drug delivery to overcome cancer multidrug resistance. Adv Mater. 2016;28(42):9341–9348. doi: 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- 219.Moratz J, Samanta A, Voskuhl J, Mohan Nalluri SK, Ravoo BJ. Light-triggered capture and release of DNA and proteins by host-guest binding and electrostatic interaction. Chemistry. 2015;21(8):3271–3277. doi: 10.1002/chem.201405936. [DOI] [PubMed] [Google Scholar]
- 220.National Toxicology P. Bioassay of azobenzene for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser. 1979;154:1–131. [PubMed] [Google Scholar]
- 221.Leung SJ, Kachur XM, Bobnick MC, Romanowski M. Wavelength-selective light-induced release from plasmon resonant liposomes. Adv Func Mater. 2011;21(6):1113–1121. doi: 10.1002/adfm.201002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mathiyazhakan M, Wiraja C, Xu C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nano-micro Lett. 2018;10(1):10. doi: 10.1007/s40820-017-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Florentsen CD, West AV, Danielsen HMD, Semsey S, Bendix PM, Oddershede LB. Quantification of loading and laser-assisted release of RNA from single gold nanoparticles. Langmuir ACS J Surf Colloids. 2018;34(49):14891–14898. doi: 10.1021/acs.langmuir.8b01831. [DOI] [PubMed] [Google Scholar]
- 224.Zhang P, Wang C, Zhao J, Xiao A, Shen Q, Li L, et al. Near infrared-guided smart nanocarriers for MicroRNA-controlled release of doxorubicin/siRNA with intracellular ATP as fuel. ACS Nano. 2016;10(3):3637–3647. doi: 10.1021/acsnano.5b08145. [DOI] [PubMed] [Google Scholar]
- 225.Iturrioz-Rodriguez N, Correa-Duarte MA, Fanarraga ML. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int J Nanomed. 2019;14:3389–3401. doi: 10.2147/IJN.S198848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang F, Zhang L, Bai X, Cao X, Jiao X, Huang Y, et al. Stimuli-responsive nanocarrier for co-delivery of MiR-31 and doxorubicin to suppress high MtEF4 cancer. ACS Appl Mater Interfaces. 2018;10(26):22767–22775. doi: 10.1021/acsami.8b07698. [DOI] [PubMed] [Google Scholar]
- 227.Ma N, Li Y, Xu H, Wang Z, Zhang X. Dual redox responsive assemblies formed from diselenide block copolymers. J Am Chem Soc. 2010;132(2):442–443. doi: 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- 228.Deng X, Yin Z, Lu J, Li X, Shao L, Zhao C, et al. In situ monitoring of MicroRNA replacement efficacy and accurate imaging-guided cancer therapy through light-up inter-polyelectrolyte nanocomplexes. Adv Sci. 2018;5(4):1700542. doi: 10.1002/advs.201700542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kang Y, Lu L, Lan J, Ding Y, Yang J, Zhang Y, et al. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018;68:137–153. doi: 10.1016/j.actbio.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 230.Ulijn VR. Enzyme-responsive materials: a new class of smart biomaterials. J Mater Chem. 2006;16:2217–2225. doi: 10.1039/b601776m. [DOI] [Google Scholar]
- 231.Li L, Wang J, Kong H, Zeng Y, Liu G. Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment. Sci Technol Adv Mater. 2018;19(1):771–790. doi: 10.1080/14686996.2018.1528850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35(13):4213–4222. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu X, Tan N, Zhou Y, Wei H, Ren S, Yu F, et al. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int J Nanomed. 2017;12:7089–7101. doi: 10.2147/IJN.S124584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wu K, Song W, Zhao L, Liu M, Yan J, Andersen MO, et al. MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity. ACS Appl Mater Interfaces. 2013;5(7):2733–2744. doi: 10.1021/am400374c. [DOI] [PubMed] [Google Scholar]
- 235.Wang Z, Wu G, Feng Z, Bai S, Dong Y, Wu G, et al. Microarc-oxidized titanium surfaces functionalized with microRNA-21-loaded chitosan/hyaluronic acid nanoparticles promote the osteogenic differentiation of human bone marrow mesenchymal stem cells. Int J Nanomed. 2015;10:6675–6687. doi: 10.2147/IJN.S94689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sahle FF, Gulfam M, Lowe TL. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov Today. 2018;23(5):992–1006. doi: 10.1016/j.drudis.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, et al. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat Commun. 2016;7:12868. doi: 10.1038/ncomms12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Griffin S, Masood MI, Nasim MJ, Sarfraz M, Ebokaiwe AP, Schafer KH, et al. Natural nanoparticles: a particular matter inspired by nature. Antioxidants. 2017;7(1):3. doi: 10.3390/antiox7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]