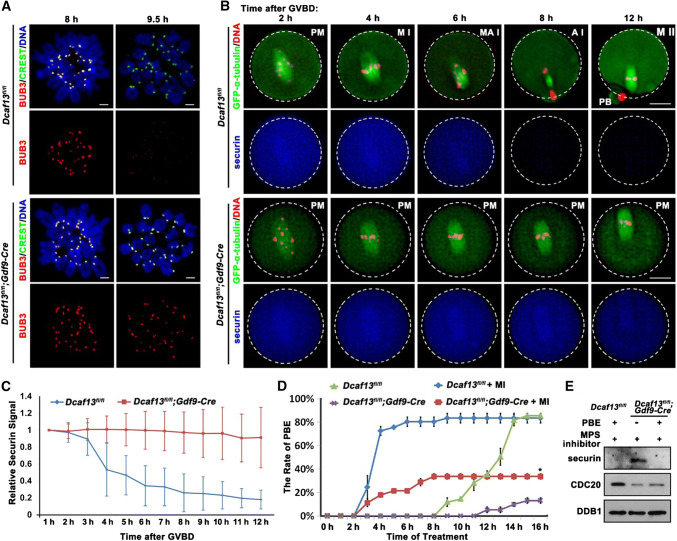
Fig. 3.
Role of spindle assembly checkpoint in Pro-MI arrest caused by Dcaf13 deletion in oocyte. a Immunofluorescence results of BUB3 (red) and CREST (green) on chromosome spreads made from Dcaf13fl/fl and Dcaf13fl/fl; Gdf9-Cre oocytes at 8 and 9.5 h after culture. Scale bar, 10 μm. b GV-arrested oocytes harvested from Dcaf13fl/fl and Dcaf13fl/fl; Gdf9-Cre mice were co-microinjected with GFP-α-tubulin (to label spindles) and mCherry-securin mRNA. After incubation in M16 containing milrinone for 8 h, microinjected oocytes were transferred to milrinone-free M16 containing Hoechst-33342 (to label DNA), and time-lapse confocal microscopy movies were recorded after GVBD. PM pre-metaphase I, MI metaphase I, M–AI metaphase–anaphase transition, AI anaphase I, MII metaphase II. Scale bar, 25 μm. c Quantification of mCherry-securin fluorescence intensity levels in WT (blue) and Dcaf13-deleted (red) oocytes at each time point. Time after GVBD is indicated. Values from individual oocytes were normalized relative to that at 1 h. Error bars indicate SEM. d PBE rates of Dcaf13fl/fl and Dcaf13fl/fl;Gdf9-Cre oocytes which were cultured with or without MPS inhibitor (10 μM) after GVBD. *P < 0.05 by two-tailed Student’s t tests. Error bars indicate SEM. e Expressed levels of securin and CDC20 in Dcaf13fl/fl and Dcaf13fl/fl;Gdf9-Cre oocytes as revealed by western blot. Samples of 100 oocytes were collected after MPS inhibitor treatment for 8 h. Levels of DDB1 were blotted as a loading control