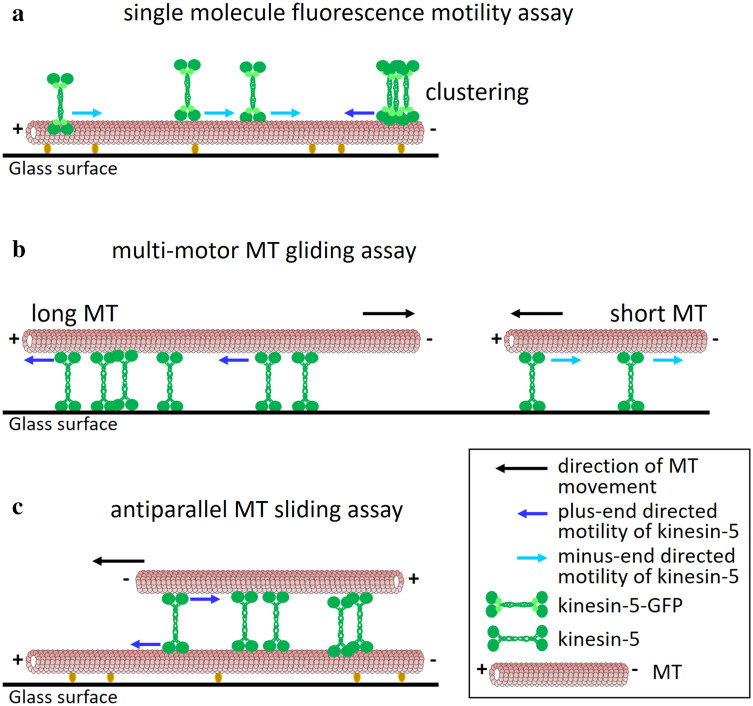
Fig. 2.
Different directionalities of kinesin-5 motors in the various types of motility assays. a Single molecule fluorescence motility assay. Fluorescently labeled MTs are immobilized on a glass surface and fluorescently labeled kinesin-5 motors [usually with green fluorescent protein (GFP)] move on the immobilized MTs, likely by interactions of one of the two pairs of catalytic domains with the immobilized MT. In such an assay, Cin8, Kip1 (in high ionic strength conditions) and Cut7 were shown to be minus end-directed [79, 106, 107, 119]. Accumulation of Cin8 in clusters on a single MT was shown to reverse directionality to plus end-directed motility [120]. b Multi-motor MT gliding assay. Motor proteins are immobilized to a glass surface and fluorescently labeled MTs undergo kinesin-5-driven motility. The directionality of the MTs is opposite to that of the immobilized kinesin-5 motors. When using long MTs in such an assay, Cin8 and Kip1 were shown to be plus end-directed [80, 106, 107, 115], while Cut7 was shown to be minus end-directed [119]. With shorter MTs, Cin8 exhibited minus end-directed motility [107]. c Antiparallel MT sliding assay. One set of MTs is immobilized to the surface while the other set, sometimes differently labeled, is free to float in solution. The free MTs undergo antiparallel sliding on top of the immobilized MTs, mediated by kinesin-5 motors that crosslink and walk on both MTs [58]. In such an assay, Cin8 was shown to undergo plus end-directed motility [106, 107, 120], similarly to kinesin-5 motors in higher eukaryotes [58, 116]