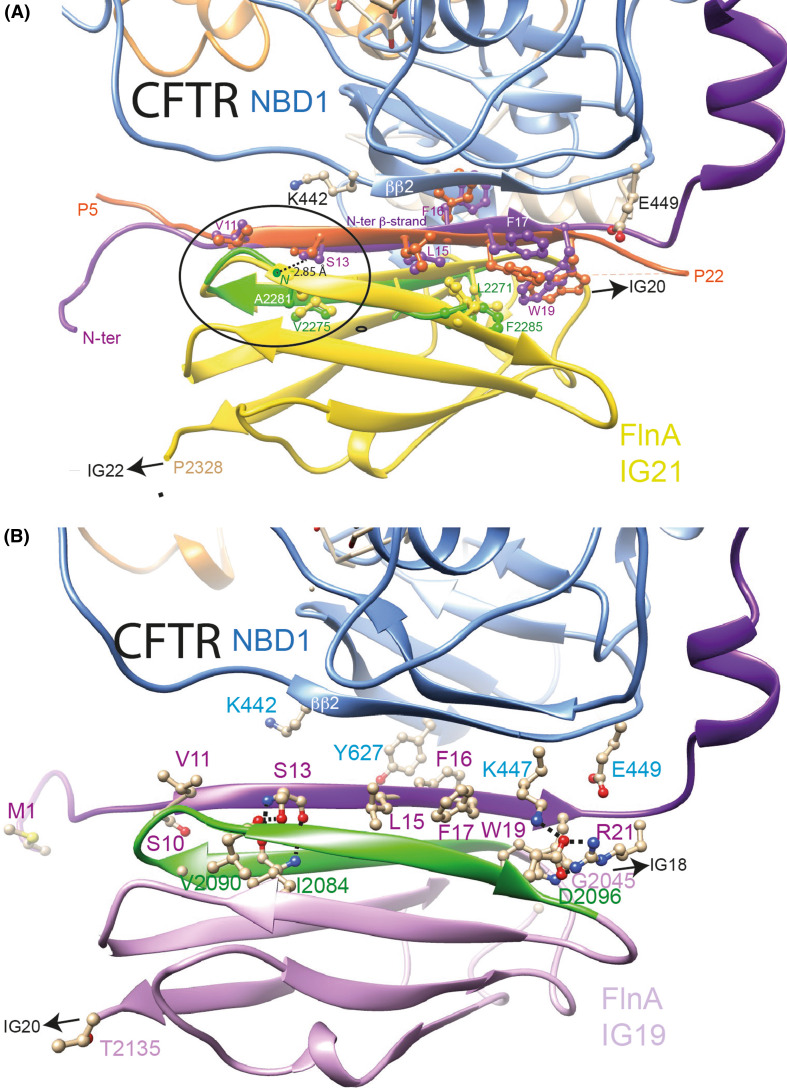
Fig. 10.
Models of human filamin in complex with CFTR N-terminus (β-strand conformation). a Complex of the CFTR open form (model) with the IG21 domain of FlnA. The analysis of the crystal structure of the FlnA IG21 (yellow) in complex with the CFTR N-terminus (peptide 5–22, orange) (PDBID:3ISW [73]) suggested that this last region of CFTR, observed in a β-strand conformation (and thus designated here as the N-ter β-strand), may form a β-sheet with NBD1 partners (strand ββ2, light blue). The CFTR 3D structure, in which the N-terminal region was modeled based on this observation, in complex with the FlnA IG21 domain, was then submitted to molecular dynamics (20 ns), leading to observe a longer N-ter β-strand (purple), which was stabilized by a strong interaction between S13 OG and A2281N atom. The green ribbon represents the FlnA IG21 β-hairpin 2271–2285 after MD simulations (RMSD between the two N-ter β-strands (orange (IG21/CFTR peptide complex, PDBID:3ISW [73]) and purple (full-length CFTR/IG21 complex after MD) and the two β-hairpin 2271-2285 (yellow (IG21/CFTR peptide complex) and green [full-length CFTR/IG21 complex after MD)] = 1.19 Å (24 Cα)). The circle indicates the region where the best superimposition is observed. b Complex of the CFTR open form (model) with the IG19 domain of FlnA. MD simulation (50 ns) was performed on the complex between IG19 (docked as IG21 on the CFTR N-ter β-strand) and CFTR. IG19 remained stable along the simulation (RMSD of 1.95 Å for 193 Cα atoms (IG19 + aa 1–22 of CFTR) between 0 and 10 ns). Among others, an H-bond is present between S13 hydroxyl and the V1290 carbonyl oxygen, as well as a salt bridge between FlnA D2096 and CFTR R21 and K447